Race/Ethnicity and the Pharmacogenetics of Reported Suicidality With Efavirenz Among Clinical Trials Participants
- PMID: 28931220
- PMCID: PMC5853681
- DOI: 10.1093/infdis/jix248
Race/Ethnicity and the Pharmacogenetics of Reported Suicidality With Efavirenz Among Clinical Trials Participants
Abstract
Background: We examined associations between suicidality and genotypes that predict plasma efavirenz exposure among AIDS Clinical Trials Group study participants in the United States.
Methods: Four clinical trials randomly assigned treatment-naive participants to efavirenz-containing regimens; suicidality was defined as reported suicidal ideation or attempted or completed suicide. Genotypes that predict plasma efavirenz exposure were defined by CYP2B6 and CYP2A6 polymorphisms. Associations were evaluated with weighted Cox proportional hazards models stratified by race/ethnicity. Additional analyses adjusted for genetic ancestry and selected covariates.
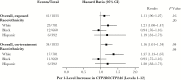
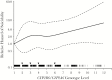
Results: Among 1833 participants, suicidality was documented in 41 in exposed analyses, and 34 in on-treatment analyses. In unadjusted analyses based on 12 genotype levels, suicidality increased per level in exposed (hazard ratio, 1.11; 95% confidence interval, .96-1.27) and on-treatment 1.16; 1.01-1.34) analyses. In the on-treatment analysis, the association was strongest among white but nearly null among black participants. Considering 3 metabolizer levels (extensive, intermediate and slow), slow metabolizers were at increased risk. Results were similar after baseline covariate-adjustment for genetic ancestry, sex, age, weight, injection drug use history, and psychiatric history or recent psychoactive medication.
Conclusions: Genotypes that predict higher plasma efavirenz exposure were associated with increased risk of suicidality. Strength of association varied by race/ethnicity.
Keywords: CYP2B6; HIV; efavirenz; pharmacogenetics; suicidality.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Similar articles
-
Secondary metabolism pathway polymorphisms and plasma efavirenz concentrations in HIV-infected adults with CYP2B6 slow metabolizer genotypes.J Antimicrob Chemother. 2014 Aug;69(8):2175-82. doi: 10.1093/jac/dku110. Epub 2014 Apr 11. J Antimicrob Chemother. 2014. PMID: 24729586 Free PMC article. Clinical Trial.
-
Pharmacogenetics of plasma efavirenz exposure in HIV-infected adults and children in South Africa.Br J Clin Pharmacol. 2015 Jul;80(1):146-56. doi: 10.1111/bcp.12590. Epub 2015 May 28. Br J Clin Pharmacol. 2015. PMID: 25611810 Free PMC article.
-
Association between efavirenz as initial therapy for HIV-1 infection and increased risk for suicidal ideation or attempted or completed suicide: an analysis of trial data.Ann Intern Med. 2014 Jul 1;161(1):1-10. doi: 10.7326/M14-0293. Ann Intern Med. 2014. PMID: 24979445 Free PMC article.
-
Pharmacogenetics of CYP2B6, CYP2A6 and UGT2B7 in HIV treatment in African populations: focus on efavirenz and nevirapine.Drug Metab Rev. 2015 May;47(2):111-23. doi: 10.3109/03602532.2014.982864. Epub 2014 Nov 13. Drug Metab Rev. 2015. PMID: 25391641 Review.
-
PharmGKB summary: Efavirenz pathway, pharmacokinetics.Pharmacogenet Genomics. 2015 Jul;25(7):363-76. doi: 10.1097/FPC.0000000000000145. Pharmacogenet Genomics. 2015. PMID: 25966836 Free PMC article. Review. No abstract available.
Cited by
-
Suicide risk among persons living with HIV.AIDS Care. 2021 May;33(5):616-622. doi: 10.1080/09540121.2020.1801982. Epub 2020 Aug 3. AIDS Care. 2021. PMID: 32741212 Free PMC article.
-
Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2B6 and Efavirenz-Containing Antiretroviral Therapy.Clin Pharmacol Ther. 2019 Oct;106(4):726-733. doi: 10.1002/cpt.1477. Epub 2019 Jul 5. Clin Pharmacol Ther. 2019. PMID: 31006110 Free PMC article.
-
Rates of suicidal ideation among HIV-infected patients in care in the HIV Outpatient Study 2000-2017, USA.Prev Med. 2020 May;134:106011. doi: 10.1016/j.ypmed.2020.106011. Epub 2020 Feb 3. Prev Med. 2020. PMID: 32027915 Free PMC article.
-
Common antiretroviral combinations are associated with somatic depressive symptoms in women with HIV.AIDS. 2024 Feb 1;38(2):167-176. doi: 10.1097/QAD.0000000000003730. Epub 2023 Sep 28. AIDS. 2024. PMID: 37773048 Free PMC article.
-
Drug metabolism and transport gene polymorphisms and efavirenz adverse effects in Brazilian HIV-positive individuals.J Antimicrob Chemother. 2018 Sep 1;73(9):2460-2467. doi: 10.1093/jac/dky190. J Antimicrob Chemother. 2018. PMID: 29868865 Free PMC article.
References
-
- van Leth F, Phanuphak P, Ruxrungtham K, et al. ; 2NN Study team Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet 2004; 363:1253–63. - PubMed
-
- Gulick RM, Ribaudo HJ, Shikuma CM, et al. ; AIDS Clinical Trials Group (ACTG) A5095 Study Team Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized controlled trial. JAMA 2006; 296:769–81. - PubMed
-
- Lennox JL, DeJesus E, Lazzarin A, et al. ; STARTMRK investigators Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 2009; 374:796–806. - PubMed
-
- Cooper DA, Heera J, Goodrich J, et al. Maraviroc versus efavirenz, both in combination with zidovudine-lamivudine, for the treatment of antiretroviral-naive subjects with CCR5-tropic HIV-1 infection. J Infect Dis 2010; 201:803–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

