A cluster-randomized trial to reduce major perinatal morbidity among women with one prior cesarean delivery in Québec (PRISMA trial): study protocol for a randomized controlled trial
- PMID: 28931404
- PMCID: PMC5608183
- DOI: 10.1186/s13063-017-2150-x
A cluster-randomized trial to reduce major perinatal morbidity among women with one prior cesarean delivery in Québec (PRISMA trial): study protocol for a randomized controlled trial
Abstract
Background: Rates of cesarean delivery are continuously increasing in industrialized countries, with repeated cesarean accounting for about a third of all cesareans. Women who have undergone a first cesarean are facing a difficult choice for their next pregnancy, i.e.: (1) to plan for a second cesarean delivery, associated with higher risk of maternal complications than vaginal delivery; or (b) to have a trial of labor (TOL) with the aim to achieve a vaginal birth after cesarean (VBAC) and to accept a significant, but rare, risk of uterine rupture and its related maternal and neonatal complications. The objective of this trial is to assess whether a multifaceted intervention would reduce the rate of major perinatal morbidity among women with one prior cesarean.
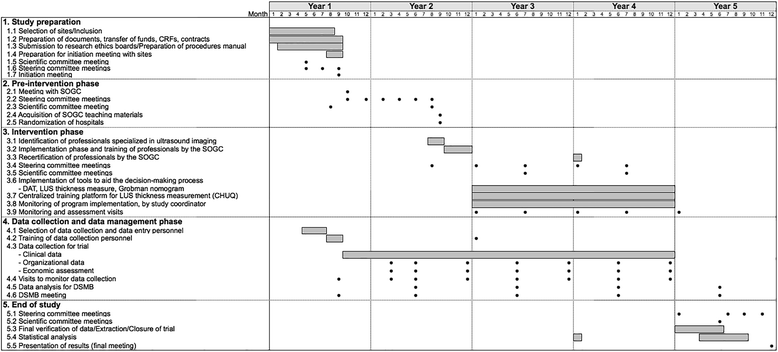
Methods/design: The study is a stratified, non-blinded, cluster-randomized, parallel-group trial of a multifaceted intervention. Hospitals in Quebec are the units of randomization and women are the units of analysis. As depicted in Figure 1, the study includes a 1-year pre-intervention period (baseline), a 5-month implementation period, and a 2-year intervention period. At the end of the baseline period, 20 hospitals will be allocated to the intervention group and 20 to the control group, using a randomization stratified by level of care. Medical records will be used to collect data before and during the intervention period. Primary outcome is the rate of a composite of major perinatal morbidities measured during the intervention period. Secondary outcomes include major and minor maternal morbidity; minor perinatal morbidity; and TOL and VBAC rate. The effect of the intervention will be assessed using the multivariable generalized-estimating-equations extension of logistic regression. The evaluation will include subgroup analyses for preterm and term birth, and a cost-effectiveness analysis.
Discussion: The intervention is designed to facilitate: (1) women's decision-making process, using a decision analysis tool (DAT), (2) an estimate of uterine rupture risk during TOL using ultrasound evaluation of low-uterine segment thickness, (3) an estimate of chance of TOL success, using a validated prediction tool, and (4) the implementation of best practices for intrapartum management.
Trial registration: Current Controlled Trials, ID: ISRCTN15346559 . Registered on 20 August 2015.
Keywords: Intrapartum management; RCT; Uterine rupture; VBAC.
Conflict of interest statement
Consent for publication
All authors have consented to publication of this article.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Public Health Agency of Canada. Canadian perinatal health report. Ottawa; 2008.
-
- Health system performance: health indicators. Ottawa: CIHI, 2005.
-
- Canadian Institute for Health Information. Giving birth in Canada: regional trends from 2001-2002 to 2005-2006. Ottawa; 2007.
-
- Allen VM, O'Connell CM, Liston RM, Baskett TF. Maternal morbidity associated with cesarean delivery without labor compared with spontaneous onset of labor at term. Obstet Gynecol. 2003;102:477–82. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials