Molecular characterization of the duck enteritis virus US10 protein
- PMID: 28931412
- PMCID: PMC5607491
- DOI: 10.1186/s12985-017-0841-2
Molecular characterization of the duck enteritis virus US10 protein
Abstract
Background: There is little information regarding the duck enteritis virus (DEV) US10 gene and its molecular characterization.
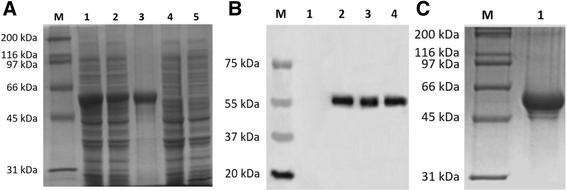
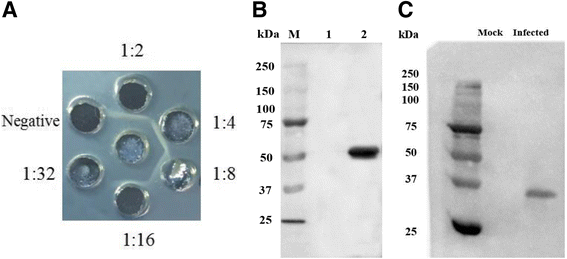
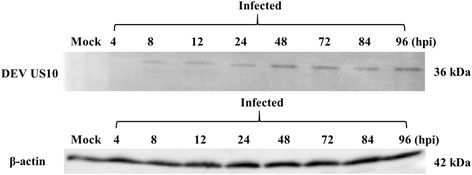
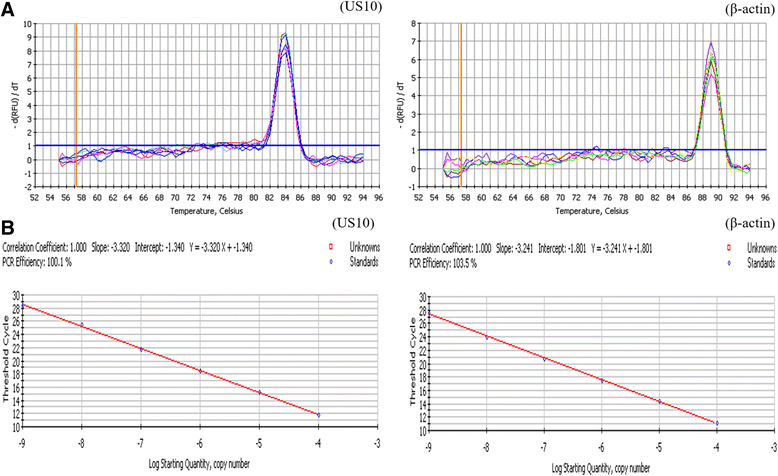
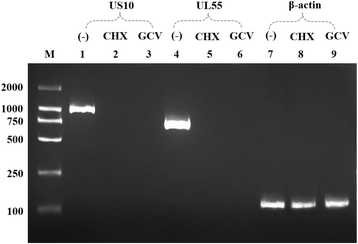
Methods: Duck enteritis virus US10 was amplified and cloned into the recombinant vector pET32a(+). The recombinant US10 protein was expressed in Escherichia coli BL21 cells and used to immunize rabbits for the preparation of polyclonal antibodies. The harvested rabbit antiserum against DEV US10 was detected and analyzed by agar immunodiffusion. Using this antibody, western blotting and indirect immunofluorescence analysis were used to analyze the expression level and subcellular localization of US10 in infected cells at different time points. Quantitative reverse-transcription PCR (qRT-PCR) and pharmacological inhibition tests were used to ascertain the kinetic class of the US10 gene. A mass spectrometry-based strategy was used to identify US10 in purified DEV virions and quantify its abundance.
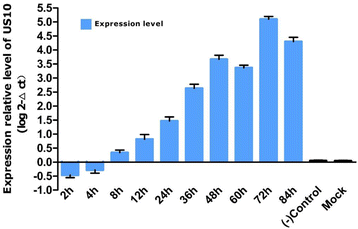
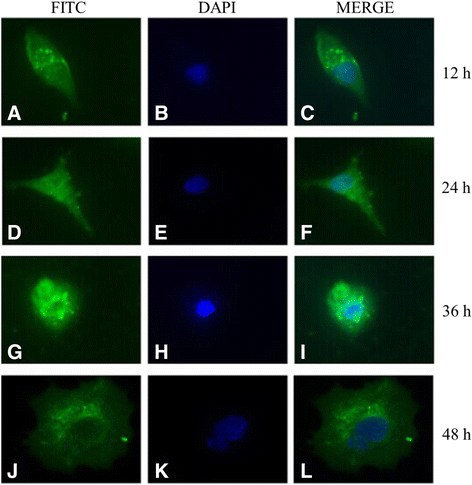
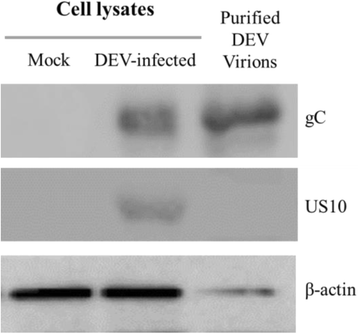
Results: The recombinant pET32a(+)/US10 protein was expressed as inclusion bodies, purified by gradient urea washing, and used to prepare specific antibodies. The results of qRT-PCR, western blotting, and pharmacological inhibition tests revealed that US10 is mainly transcribed in the late stage of viral replication. However, the presence of the DNA polymerase inhibitor ganciclovir and the protein synthesis inhibitor cycloheximide blocked transcription. Therefore, US10 is a γ2 (true late) gene. Indirect immunofluorescence analysis showed that US10 proteins were initially diffusely distributed throughout the cytoplasm, but with the passage of time, they gradually relocated to a perinuclear region. The US10 protein was detected in purified DEV virions by mass spectrometry, but was not detected by western blotting, indicating that DEV US10 is a minor virion protein.
Conclusions: The DEV US10 gene is a γ2 gene and the US10 protein is localized in the perinuclear region. DEV US10 is a virion component.
Keywords: Duck enteritis virus; Kinetic class; Subcellular localization; True late gene; US10; Virion protein; γ2 Gene.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Animal Ethics Committee of Sichuan Agricultural University (2016-17).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Molecular characterization of duck enteritis virus UL41 protein.Virol J. 2018 Jan 15;15(1):12. doi: 10.1186/s12985-018-0928-4. Virol J. 2018. PMID: 29334975 Free PMC article.
-
Identification and characterization of the duck enteritis virus (DEV) US2 gene.Genet Mol Res. 2015 Oct 29;14(4):13779-90. doi: 10.4238/2015.October.28.40. Genet Mol Res. 2015. PMID: 26535693
-
Duck enteritis virus UL54 is an IE protein primarily located in the nucleus.Virol J. 2015 Nov 25;12:198. doi: 10.1186/s12985-015-0424-z. Virol J. 2015. PMID: 26606920 Free PMC article.
-
Replication kinetics of duck enteritis virus UL16 gene in vitro.Virol J. 2012 Nov 21;9:281. doi: 10.1186/1743-422X-9-281. Virol J. 2012. PMID: 23171438 Free PMC article.
-
Current status of recombinant duck enteritis virus vector vaccine research.Front Vet Sci. 2025 Feb 5;12:1453150. doi: 10.3389/fvets.2025.1453150. eCollection 2025. Front Vet Sci. 2025. PMID: 39974164 Free PMC article. Review.
Cited by
-
The Pivotal Roles of US3 Protein in Cell-to-Cell Spread and Virion Nuclear Egress of Duck Plague Virus.Sci Rep. 2020 Apr 28;10(1):7181. doi: 10.1038/s41598-020-64190-2. Sci Rep. 2020. PMID: 32346128 Free PMC article.
-
Duplicate US1 Genes of Duck Enteritis Virus Encode a Non-essential Immediate Early Protein Localized to the Nucleus.Front Cell Infect Microbiol. 2020 Jan 17;9:463. doi: 10.3389/fcimb.2019.00463. eCollection 2019. Front Cell Infect Microbiol. 2020. PMID: 32010642 Free PMC article.
-
Duck Enteritis Virus VP16 Antagonizes IFN-β-Mediated Antiviral Innate Immunity.J Immunol Res. 2020 May 15;2020:9630452. doi: 10.1155/2020/9630452. eCollection 2020. J Immunol Res. 2020. PMID: 32537474 Free PMC article.
-
Characterization of a Unique Novel LORF3 Protein of Duck Plague Virus and Its Potential Pathogenesis.J Virol. 2023 Jan 31;97(1):e0157722. doi: 10.1128/jvi.01577-22. Epub 2023 Jan 4. J Virol. 2023. PMID: 36598202 Free PMC article.
-
Duck enteritis virus pUL47, as a late structural protein localized in the nucleus, mainly depends on residues 40 to 50 and 768 to 777 and inhibits IFN-β signalling by interacting with STAT1.Vet Res. 2020 Nov 11;51(1):135. doi: 10.1186/s13567-020-00859-w. Vet Res. 2020. PMID: 33176874 Free PMC article.
References
-
- Samia AM. Viral infections of waterfowl: duck virus enteritis (duck plague) In: Swayne DE, Glisson JR, Mcdougald LR, Nolan LK, Suarez DL, Nair VL, editors. Diseases of poultry. 13. Oxford: Wiley-Blackwell; 2013. pp. 431–440.
-
- Baudet A. Mortality in ducks in the Netherlands caused by a filtrable virus; fowl plague. Tijdschr Diergeneeskd. 1923;50:455–459.
-
- International Committee on Taxonomy of Viruses (ICTV) : The Online (10th) Report of the ICTV.https://talk.ictvonline.org/ictv-reports/ictv_online_report (2015). Accessed July 2015.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

