DNA Priming Increases Frequency of T-Cell Responses to a Vesicular Stomatitis Virus HIV Vaccine with Specific Enhancement of CD8+ T-Cell Responses by Interleukin-12 Plasmid DNA
- PMID: 28931520
- PMCID: PMC5674193
- DOI: 10.1128/CVI.00263-17
DNA Priming Increases Frequency of T-Cell Responses to a Vesicular Stomatitis Virus HIV Vaccine with Specific Enhancement of CD8+ T-Cell Responses by Interleukin-12 Plasmid DNA
Abstract
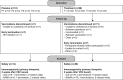
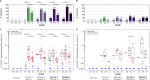
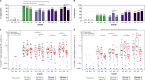
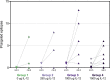
The HIV Vaccine Trials Network (HVTN) 087 vaccine trial assessed the effect of increasing doses of pIL-12 (interleukin-12 delivered as plasmid DNA) adjuvant on the immunogenicity of an HIV-1 multiantigen (MAG) DNA vaccine delivered by electroporation and boosted with a vaccine comprising an attenuated vesicular stomatitis virus expressing HIV-1 Gag (VSV-Gag). We randomized 100 healthy adults to receive placebo or 3 mg HIV-MAG DNA vaccine (ProfectusVax HIV-1 gag/pol or ProfectusVax nef/tat/vif, env) coadministered with pIL-12 at 0, 250, 1,000, or 1,500 μg intramuscularly by electroporation at 0, 1, and 3 months followed by intramuscular inoculation with 3.4 × 107 PFU VSV-Gag vaccine at 6 months. Immune responses were assessed after the prime and boost and 6 months after the last vaccination. High-dose pIL-12 increased the magnitude of CD8+ T-cell responses postboost compared to no pIL-12 (P = 0.02), while CD4+ T-cell responses after the prime were higher in the absence of pIL-12 than with low- and medium-dose pIL-12 (P ≤ 0.05). The VSV boost increased Gag-specific CD4+ and CD8+ T-cell responses in all groups (P < 0.001 for CD4+ T cells), inducing a median of four Gag epitopes in responders. Six to 9 months after the boost, responses decreased in magnitude, but CD8+ T-cell response rates were maintained. The addition of a DNA prime dramatically improved responses to the VSV vaccine tested previously in the HVTN 090 trial, leading to broad epitope targeting and maintained CD8+ T-cell response rates at early memory. The addition of high-dose pIL-12 given with a DNA prime by electroporation and boosted with VSV-Gag increased the CD8+ T-cell responses but decreased the CD4+ responses. This approach may be advantageous in reshaping the T-cell responses to a variety of chronic infections or tumors. (This study has been registered at ClinicalTrials.gov under registration no. NCT01578889.).
Keywords: DNA prime; HIV; IL-12; T cell; VSV vector; vaccine.
Copyright © 2017 American Society for Microbiology.
Figures

References
-
- UNAIDS. 2014. The gap report. UNAIDS, Geneva, Switzerland: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_....
-
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi:10.1056/NEJMoa1113425. - DOI - PMC - PubMed
-
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M Jr, Lifson JD, Picker LJ. 2011. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473:523–527. doi:10.1038/nature10003. - DOI - PMC - PubMed
-
- Hansen SG, Piatak M Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, Gilliam AN, Xu G, Whizin N, Burwitz BJ, Planer SL, Turner JM, Legasse AW, Axthelm MK, Nelson JA, Fruh K, Sacha JB, Estes JD, Keele BF, Edlefsen PT, Lifson JD, Picker LJ. 2013. Immune clearance of highly pathogenic SIV infection. Nature 502:100–104. doi:10.1038/nature12519. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

