Cellubrevin/vesicle-associated membrane protein-3-mediated endocytosis and trafficking regulate platelet functions
- PMID: 28931526
- PMCID: PMC5746669
- DOI: 10.1182/blood-2017-02-768176
Cellubrevin/vesicle-associated membrane protein-3-mediated endocytosis and trafficking regulate platelet functions
Abstract
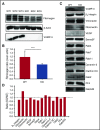
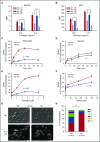
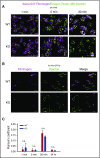
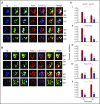
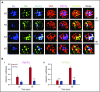
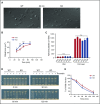
Endocytosis is key to fibrinogen (Fg) uptake, trafficking of integrins (αIIbβ3, αvβ3), and purinergic receptors (P2Y1, P2Y12), and thus normal platelet function. However, the molecular machinery required and possible trafficking routes are still ill-defined. To further identify elements of the platelet endocytic machinery, we examined the role of a vesicle-residing, soluble N-ethylmaleimide factor attachment protein receptor (v-SNARE) called cellubrevin/vesicle-associated membrane protein-3 (VAMP-3) in platelet function. Although not required for normal platelet exocytosis or hemostasis, VAMP-3-/- mice had less platelet-associated Fg, indicating a defect in Fg uptake/storage. Other granule markers were unaffected. Direct experiments, both in vitro and in vivo, showed that loss of VAMP-3 led to a robust defect in uptake/storage of Fg in platelets and cultured megakaryocytes. Uptake of the fluid-phase marker, dextran, was only modestly affected. Time-dependent uptake and endocytic trafficking of Fg and dextran were followed using 3-dimensional-structured illumination microscopy. Dextran uptake was rapid compared with Fg, but both cargoes progressed through Rab4+, Rab11+, and von Willebrand factor (VWF)+ compartments in wild-type platelets in a time-dependent manner. In VAMP-3-/- platelets, the 2 cargoes showed limited colocalization with Rab4, Rab11, or VWF. Loss of VAMP-3 also affected some acute platelet functions, causing enhanced spreading on Fg and fibronectin and faster clot retraction compared with wild-type. In addition, the rate of Janus kinase 2 phosphorylation, initiated through the thrombopoietin receptor (TPOR/Mpl) activation, was affected in VAMP-3-/- platelets. Collectively, our studies show that platelets are capable of a range of endocytosis steps, with VAMP-3 being pivotal in these processes.
© 2017 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
Comment in
-
VAMP-3 mediates platelet endocytosis.Blood. 2017 Dec 28;130(26):2816-2818. doi: 10.1182/blood-2017-10-808576. Blood. 2017. PMID: 29284612 Free PMC article.
Similar articles
-
Arf6 controls platelet spreading and clot retraction via integrin αIIbβ3 trafficking.Blood. 2016 Mar 17;127(11):1459-67. doi: 10.1182/blood-2015-05-648550. Epub 2016 Jan 6. Blood. 2016. PMID: 26738539 Free PMC article.
-
NBEAL2 (Neurobeachin-Like 2) Is Required for Retention of Cargo Proteins by α-Granules During Their Production by Megakaryocytes.Arterioscler Thromb Vasc Biol. 2018 Oct;38(10):2435-2447. doi: 10.1161/ATVBAHA.118.311270. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354215
-
Granule stores from cellubrevin/VAMP-3 null mouse platelets exhibit normal stimulus-induced release.Blood. 2003 Sep 1;102(5):1716-22. doi: 10.1182/blood-2003-01-0331. Epub 2003 May 8. Blood. 2003. PMID: 12738662
-
Small GTPases in platelet membrane trafficking.Platelets. 2019;30(1):31-40. doi: 10.1080/09537104.2018.1535703. Epub 2018 Oct 26. Platelets. 2019. PMID: 30365369 Free PMC article. Review.
-
The ins and outs of endocytic trafficking in platelet functions.Curr Opin Hematol. 2017 Sep;24(5):467-474. doi: 10.1097/MOH.0000000000000366. Curr Opin Hematol. 2017. PMID: 28650849 Free PMC article. Review.
Cited by
-
Platelet-HIV: interactions and their implications.Platelets. 2022 Feb 17;33(2):208-211. doi: 10.1080/09537104.2021.2019695. Epub 2022 Jan 4. Platelets. 2022. PMID: 35086429 Free PMC article.
-
SYPL1 defines a vesicular pathway essential for sperm cytoplasmic droplet formation and male fertility.Nat Commun. 2023 Aug 22;14(1):5113. doi: 10.1038/s41467-023-40862-1. Nat Commun. 2023. PMID: 37607933 Free PMC article.
-
Platelets and Immunity: Going Viral.Arterioscler Thromb Vasc Biol. 2020 Jul;40(7):1605-1607. doi: 10.1161/ATVBAHA.120.314620. Epub 2020 Jun 24. Arterioscler Thromb Vasc Biol. 2020. PMID: 32579477 Free PMC article. No abstract available.
-
Platelet Toll-Like-Receptor-2 and -4 Mediate Different Immune-Related Responses to Bacterial Ligands.TH Open. 2022 Jul 11;6(3):e156-e167. doi: 10.1055/a-1827-7365. eCollection 2022 Jul. TH Open. 2022. PMID: 36046205 Free PMC article.
-
Components of the endocytic and recycling trafficking pathways interfere with the integrity of the Legionella-containing vacuole.Cell Microbiol. 2020 Apr;22(4):e13151. doi: 10.1111/cmi.13151. Cell Microbiol. 2020. PMID: 32096265 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

