Cancer-associated fibroblasts lead tumor invasion through integrin-β3-dependent fibronectin assembly
- PMID: 28931556
- PMCID: PMC5674886
- DOI: 10.1083/jcb.201702033
Cancer-associated fibroblasts lead tumor invasion through integrin-β3-dependent fibronectin assembly
Abstract
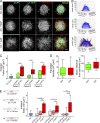
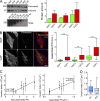
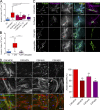
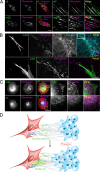
Cancer-associated fibroblasts (CAFs) are the most abundant cells of the tumor stroma. Their capacity to contract the matrix and induce invasion of cancer cells has been well documented. However, it is not clear whether CAFs remodel the matrix by other means, such as degradation, matrix deposition, or stiffening. We now show that CAFs assemble fibronectin (FN) and trigger invasion mainly via integrin-αvβ3. In the absence of FN, contractility of the matrix by CAFs is preserved, but their ability to induce invasion is abrogated. When degradation is impaired, CAFs retain the capacity to induce invasion in an FN-dependent manner. The level of expression of integrins αv and β3 and the amount of assembled FN are directly proportional to the invasion induced by fibroblast populations. Our results highlight FN assembly and integrin-αvβ3 expression as new hallmarks of CAFs that promote tumor invasion.
© 2017 Attieh et al.
Figures

References
-
- Calvo F., Ege N., Grande-Garcia A., Hooper S., Jenkins R.P., Chaudhry S.I., Harrington K., Williamson P., Moeendarbary E., Charras G., and Sahai E.. 2013. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 15:637–646. 10.1038/ncb2756 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

