Towards Precision Medicine for Hypertension: A Review of Genomic, Epigenomic, and Microbiomic Effects on Blood Pressure in Experimental Rat Models and Humans
- PMID: 28931564
- PMCID: PMC6347103
- DOI: 10.1152/physrev.00035.2016
Towards Precision Medicine for Hypertension: A Review of Genomic, Epigenomic, and Microbiomic Effects on Blood Pressure in Experimental Rat Models and Humans
Abstract
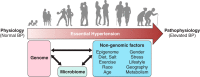
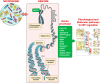
Compelling evidence for the inherited nature of essential hypertension has led to extensive research in rats and humans. Rats have served as the primary model for research on the genetics of hypertension resulting in identification of genomic regions that are causally associated with hypertension. In more recent times, genome-wide studies in humans have also begun to improve our understanding of the inheritance of polygenic forms of hypertension. Based on the chronological progression of research into the genetics of hypertension as the "structural backbone," this review catalogs and discusses the rat and human genetic elements mapped and implicated in blood pressure regulation. Furthermore, the knowledge gained from these genetic studies that provide evidence to suggest that much of the genetic influence on hypertension residing within noncoding elements of our DNA and operating through pervasive epistasis or gene-gene interactions is highlighted. Lastly, perspectives on current thinking that the more complex "triad" of the genome, epigenome, and the microbiome operating to influence the inheritance of hypertension, is documented. Overall, the collective knowledge gained from rats and humans is disappointing in the sense that major hypertension-causing genes as targets for clinical management of essential hypertension may not be a clinical reality. On the other hand, the realization that the polygenic nature of hypertension prevents any single locus from being a relevant clinical target for all humans directs future studies on the genetics of hypertension towards an individualized genomic approach.
Copyright © 2017 the American Physiological Society.
Figures


References
-
- Abiola O, Angel JM, Avner P, Bachmanov AA, Belknap JK, Bennett B, Blankenhorn EP, Blizard DA, Bolivar V, Brockmann GA, Buck KJ, Bureau JF, Casley WL, Chesler EJ, Cheverud JM, Churchill GA, Cook M, Crabbe JC, Crusio WE, Darvasi A, de Haan G, Dermant P, Doerge RW, Elliot RW, Farber CR, Flaherty L, Flint J, Gershenfeld H, Gibson JP, Gu J, Gu W, Himmelbauer H, Hitzemann R, Hsu HC, Hunter K, Iraqi FF, Jansen RC, Johnson TE, Jones BC, Kempermann G, Lammert F, Lu L, Manly KF, Matthews DB, Medrano JF, Mehrabian M, Mittlemann G, Mock BA, Mogil JS, Montagutelli X, Morahan G, Mountz JD, Nagase H, Nowakowski RS, O’Hara BF, Osadchuk AV, Paigen B, Palmer AA, Peirce JL, Pomp D, Rosemann M, Rosen GD, Schalkwyk LC, Seltzer Z, Settle S, Shimomura K, Shou S, Sikela JM, Siracusa LD, Spearow JL, Teuscher C, Threadgill DW, Toth LA, Toye AA, Vadasz C, Van Zant G, Wakeland E, Williams RW, Zhang HG, Zou F; The Complex Trait Consortium . The nature and identification of quantitative trait loci: a community’s view. Nat Rev Genet : 911–916, 2003. doi: 10.1038/nrg1206. - DOI - PMC - PubMed
-
- Aitman TJ, Glazier AM, Wallace CA, Cooper LD, Norsworthy PJ, Wahid FN, Al-Majali KM, Trembling PM, Mann CJ, Shoulders CC, Graf D, St Lezin E, Kurtz TW, Kren V, Pravenec M, Ibrahimi A, Abumrad NA, Stanton LW, Scott J. Identification of Cd36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat Genet : 76–83, 1999. doi: 10.1038/5013. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

