Micropipette force probe to quantify single-cell force generation: application to T-cell activation
- PMID: 28931600
- PMCID: PMC5687025
- DOI: 10.1091/mbc.E17-06-0385
Micropipette force probe to quantify single-cell force generation: application to T-cell activation
Abstract
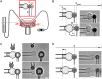
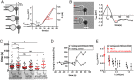
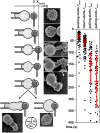
In response to engagement of surface molecules, cells generate active forces that regulate many cellular processes. Developing tools that permit gathering mechanical and morphological information on these forces is of the utmost importance. Here we describe a new technique, the micropipette force probe, that uses a micropipette as a flexible cantilever that can aspirate at its tip a bead that is coated with molecules of interest and is brought in contact with the cell. This technique simultaneously allows tracking the resulting changes in cell morphology and mechanics as well as measuring the forces generated by the cell. To illustrate the power of this technique, we applied it to the study of human primary T lymphocytes (T-cells). It allowed the fine monitoring of pushing and pulling forces generated by T-cells in response to various activating antibodies and bending stiffness of the micropipette. We further dissected the sequence of mechanical and morphological events occurring during T-cell activation to model force generation and to reveal heterogeneity in the cell population studied. We also report the first measurement of the changes in Young's modulus of T-cells during their activation, showing that T-cells stiffen within the first minutes of the activation process.
© 2017 Sawicka et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures

Similar articles
-
Inverse tissue mechanics of cell monolayer expansion.PLoS Comput Biol. 2018 Mar 1;14(3):e1006029. doi: 10.1371/journal.pcbi.1006029. eCollection 2018 Mar. PLoS Comput Biol. 2018. PMID: 29494578 Free PMC article.
-
Micropipette force sensors for in vivo force measurements on single cells and multicellular microorganisms.Nat Protoc. 2019 Feb;14(2):594-615. doi: 10.1038/s41596-018-0110-x. Nat Protoc. 2019. PMID: 30697007
-
Atomic force microscopy studies on cellular elastic and viscoelastic properties.Sci China Life Sci. 2018 Jan;61(1):57-67. doi: 10.1007/s11427-016-9041-9. Epub 2017 Jun 29. Sci China Life Sci. 2018. PMID: 28667516
-
Imaging: Gear up for mechano-immunology.Cell Immunol. 2020 Apr;350:103926. doi: 10.1016/j.cellimm.2019.103926. Epub 2019 May 16. Cell Immunol. 2020. PMID: 31151736 Review.
-
Force nanoscopy of cell mechanics and cell adhesion.Nanoscale. 2013 May 21;5(10):4094-104. doi: 10.1039/c3nr00340j. Nanoscale. 2013. PMID: 23535827 Review.
Cited by
-
Advances in Micropipette Aspiration: Applications in Cell Biomechanics, Models, and Extended Studies.Biophys J. 2019 Feb 19;116(4):587-594. doi: 10.1016/j.bpj.2019.01.004. Epub 2019 Jan 7. Biophys J. 2019. PMID: 30683304 Free PMC article. Review.
-
Multiple actin networks coordinate mechanotransduction at the immunological synapse.J Cell Biol. 2020 Feb 3;219(2):e201911058. doi: 10.1083/jcb.201911058. J Cell Biol. 2020. PMID: 31977034 Free PMC article.
-
Mechanosurveillance: Tiptoeing T Cells.Front Immunol. 2022 May 26;13:886328. doi: 10.3389/fimmu.2022.886328. eCollection 2022. Front Immunol. 2022. PMID: 35693808 Free PMC article. Review.
-
Non-invasive acquisition of mechanical properties of cells via passive microfluidic mechanisms: A review.Biomicrofluidics. 2021 Jun 14;15(3):031501. doi: 10.1063/5.0052185. eCollection 2021 May. Biomicrofluidics. 2021. PMID: 34178202 Free PMC article. Review.
-
Mechanical control of antigen detection and discrimination by T and B cell receptors.Biophys J. 2024 Aug 6;123(15):2234-2255. doi: 10.1016/j.bpj.2024.05.020. Epub 2024 May 23. Biophys J. 2024. PMID: 38794795 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

