Sublethal Total Body Irradiation Causes Long-Term Deficits in Thymus Function by Reducing Lymphoid Progenitors
- PMID: 28931604
- PMCID: PMC5659725
- DOI: 10.4049/jimmunol.1600934
Sublethal Total Body Irradiation Causes Long-Term Deficits in Thymus Function by Reducing Lymphoid Progenitors
Abstract
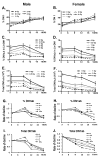
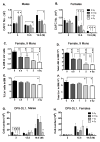
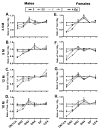
Total body irradiation (TBI) damages hematopoietic cells in the bone marrow and thymus; however, the long-term effects of irradiation with aging remain unclear. In this study, we found that the impact of radiation on thymopoiesis in mice varied by sex and dose but, overall, thymopoiesis remained suppressed for ≥12 mo after a single exposure. Male and female mice showed a long-term dose-dependent reduction in thymic cKit+ lymphoid progenitors that was maintained throughout life. Damage to hematopoietic stem cells (HSCs) in the bone marrow was dose dependent, with as little as 0.5 Gy causing a significant long-term reduction. In addition, the potential for T lineage commitment was radiation sensitive with aging. Overall, the impact of irradiation on the hematopoietic lineage was more severe in females. In contrast, the rate of decline in thymic epithelial cell numbers with age was radiation-sensitive only in males, and other characteristics including Ccl25 transcription were unaffected. Taken together, these data suggest that long-term suppression of thymopoiesis after sublethal irradiation was primarily due to fewer progenitors in the BM combined with reduced potential for T lineage commitment. A single irradiation dose also caused synchronization of thymopoiesis, with a periodic thymocyte differentiation profile persisting for at least 12 mo postirradiation. This study suggests that the number and capability of HSCs for T cell production can be dramatically and permanently damaged after a single relatively low TBI dose, accelerating aging-associated thymic involution. Our findings may impact evaluation and therapeutic intervention of human TBI events.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures




References
-
- Donskoy E, Goldschneider I. Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life. A study in parabiotic mice. J Immunol. 1992;148:1604–1612. - PubMed
-
- Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zuniga-Pflucker JC, Petrie HT. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 2004;20:735–745. - PubMed
-
- Shortman K, Wu L. Early T lymphocyte progenitors. Annu Rev Immunol. 1996;14:29–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

