Normobaric Hyperoxia Reduces Blood Occludin Fragments in Rats and Patients With Acute Ischemic Stroke
- PMID: 28931617
- PMCID: PMC5659343
- DOI: 10.1161/STROKEAHA.117.017713
Normobaric Hyperoxia Reduces Blood Occludin Fragments in Rats and Patients With Acute Ischemic Stroke
Abstract
Background and purpose: Damage of the blood-brain barrier (BBB) increases the incidence of neurovascular complications, especially for cerebral hemorrhage after tPA (tissue-type plasminogen activator) therapy. Currently, there is no effective method to evaluate the extent of BBB damage to guide tPA use. Herein, we investigated whether blood levels of tight junction proteins could serve as biomarker of BBB damages in acute ischemic stroke (AIS) in both rats and patients. We examined whether this biomarker could reflect the extent of BBB permeability during cerebral ischemia/reperfusion and the effects of normobaric hyperoxia (NBO) on BBB damage.
Methods: Rats were exposed to NBO (100% O2) or normoxia (21% O2) during middle cerebral artery occlusion. BBB permeability was determined. Occludin and claudin-5 in blood and cerebromicrovessels were measured. Patients with AIS were assigned to oxygen therapy or room air for 4 hours, and blood occludin and claudin-5 were measured at different time points after stroke.
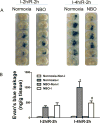
Results: Cerebral ischemia/reperfusion resulted in the degradation of occludin and claudin-5 in microvessels, leading to increased BBB permeability in rats. In blood samples, occludin increased with 4-hour ischemia and remained elevated during reperfusion, correlating well with its loss from ischemic cerebral microvessels. NBO treatment both prevented occludin degradation in microvessels and reduced occludin levels in blood, leading to improved neurological functions in rats. In patients with AIS receiving intravenous tPA thrombolysis, the blood occludin was already elevated when patients arrived at hospital (within 4.5 hours since symptoms appeared) and remained at a high level for 72 hours. NBO significantly lowered the level of blood occludin and improved neurological functions in patients with AIS.
Conclusions: Blood occludin may be a clinically viable biomarker for evaluating BBB damage during ischemia/reperfusion. NBO therapy has the potential to reduce blood occludin, protect BBB, and improve outcome in AIS patients with intravenous tPA thrombolysis.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT02974283.
Keywords: blood–brain barrier; humans; occludin; oxygen; stroke.
© 2017 American Heart Association, Inc.
Conflict of interest statement
Figures

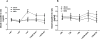
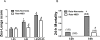
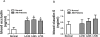
Similar articles
-
Normobaric hyperoxia slows blood-brain barrier damage and expands the therapeutic time window for tissue-type plasminogen activator treatment in cerebral ischemia.Stroke. 2015 May;46(5):1344-1351. doi: 10.1161/STROKEAHA.114.008599. Epub 2015 Mar 24. Stroke. 2015. PMID: 25804925 Free PMC article.
-
Blood Occludin Level as a Potential Biomarker for Early Blood Brain Barrier Damage Following Ischemic Stroke.Sci Rep. 2017 Jan 12;7:40331. doi: 10.1038/srep40331. Sci Rep. 2017. PMID: 28079139 Free PMC article.
-
Normobaric hyperoxia attenuates early blood-brain barrier disruption by inhibiting MMP-9-mediated occludin degradation in focal cerebral ischemia.J Neurochem. 2009 Feb;108(3):811-20. doi: 10.1111/j.1471-4159.2008.05821.x. J Neurochem. 2009. PMID: 19187098 Free PMC article.
-
Oxygen or cooling, to make a decision after acute ischemia stroke.Med Gas Res. 2016 Dec 30;6(4):206-211. doi: 10.4103/2045-9912.196902. eCollection 2016 Oct-Dec. Med Gas Res. 2016. PMID: 28217292 Free PMC article. Review.
-
Occludin regulation of blood-brain barrier and potential therapeutic target in ischemic stroke.Brain Circ. 2020 Sep 30;6(3):152-162. doi: 10.4103/bc.bc_29_20. eCollection 2020 Jul-Sep. Brain Circ. 2020. PMID: 33210038 Free PMC article. Review.
Cited by
-
Exposure to hyperbaric O2 levels leads to blood-brain barrier breakdown in rodents.Fluids Barriers CNS. 2024 May 16;21(1):41. doi: 10.1186/s12987-024-00543-7. Fluids Barriers CNS. 2024. PMID: 38755589 Free PMC article.
-
Normobaric Oxygen May Ameliorate Cerebral Venous Outflow Disturbance-Related Neurological Symptoms.Front Neurol. 2020 Nov 13;11:599985. doi: 10.3389/fneur.2020.599985. eCollection 2020. Front Neurol. 2020. PMID: 33281736 Free PMC article.
-
Blood-Brain Barrier Dysfunction in Normal Aging and Neurodegeneration: Mechanisms, Impact, and Treatments.Stroke. 2023 Mar;54(3):661-672. doi: 10.1161/STROKEAHA.122.040578. Epub 2023 Feb 27. Stroke. 2023. PMID: 36848419 Free PMC article. Review.
-
Advances in Normobaric Hyperoxia Brain Protection in Experimental Stroke.Front Neurol. 2020 Jan 31;11:50. doi: 10.3389/fneur.2020.00050. eCollection 2020. Front Neurol. 2020. PMID: 32076416 Free PMC article. Review.
-
Gut Leakage Markers and Cognitive Functions in Patients with Attention-Deficit/Hyperactivity Disorder.Children (Basel). 2023 Mar 5;10(3):513. doi: 10.3390/children10030513. Children (Basel). 2023. PMID: 36980071 Free PMC article.
References
-
- Intracerebral hemorrhage after intravenous t-pa therapy for ischemic stroke. The ninds t-pa stroke study group. Stroke. 1997;28:2109–2118. - PubMed
-
- Jin X, Liu J, Yang Y, Liu KJ, Yang Y, Liu W. Spatiotemporal evolution of blood brain barrier damage and tissue infarction within the first 3h after ischemia onset. Neurobiology of disease. 2012;48:309–316. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

