The first wave of T lymphopoiesis in zebrafish arises from aorta endothelium independent of hematopoietic stem cells
- PMID: 28931624
- PMCID: PMC5679161
- DOI: 10.1084/jem.20170488
The first wave of T lymphopoiesis in zebrafish arises from aorta endothelium independent of hematopoietic stem cells
Abstract
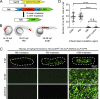
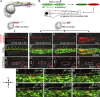
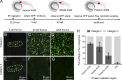
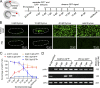
T lymphocytes are key cellular components of the adaptive immune system and play a central role in cell-mediated immunity in vertebrates. Despite their heterogeneities, it is believed that all different types of T lymphocytes are generated exclusively via the differentiation of hematopoietic stem cells (HSCs). Using temporal-spatial resolved fate-mapping analysis and time-lapse imaging, here we show that the ventral endothelium in the zebrafish aorta-gonad-mesonephros and posterior blood island, the hematopoietic tissues previously known to generate HSCs and erythromyeloid progenitors, respectively, gives rise to a transient wave of T lymphopoiesis independent of HSCs. This HSC-independent T lymphopoiesis occurs early and generates predominantly CD4 Tαβ cells in the larval but not juvenile and adult stages, whereas HSC-dependent T lymphopoiesis emerges late and produces various subtypes of T lymphocytes continuously from the larval stage to adulthood. Our study unveils the existence, origin, and ontogeny of HSC-independent T lymphopoiesis in vivo and reveals the complexity of the endothelial-hematopoietic transition of the aorta.
© 2017 Tian et al.
Figures

References
-
- Beaudin A.E., Boyer S.W., Perez-Cunningham J., Hernandez G.E., Derderian S.C., Jujjavarapu C., Aaserude E., MacKenzie T., and Forsberg E.C.. 2016. A transient developmental hematopoietic stem cell gives rise to innate-like B and T cells. Cell Stem Cell. 19:768–783. 10.1016/j.stem.2016.08.013 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

