UFM1 founder mutation in the Roma population causes recessive variant of H-ABC
- PMID: 28931644
- PMCID: PMC5664304
- DOI: 10.1212/WNL.0000000000004578
UFM1 founder mutation in the Roma population causes recessive variant of H-ABC
Abstract
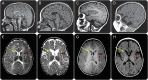
Objective: To identify the gene defect in patients with hypomyelination with atrophy of the basal ganglia and cerebellum (H-ABC) who are negative for TUBB4A mutations.
Methods: We performed homozygosity mapping and whole exome sequencing (WES) to detect the disease-causing variant. We used a Taqman assay for population screening. We developed a luciferase reporter construct to investigate the effect of the promoter mutation on expression.
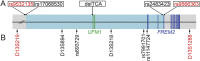
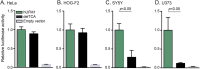
Results: Sixteen patients from 14 families from different countries fulfilling the MRI criteria for H-ABC exhibited a similar, severe clinical phenotype, including lack of development and a severe epileptic encephalopathy. The majority of patients had a known Roma ethnic background. Single nucleotide polymorphism array analysis in 5 patients identified one large overlapping homozygous region on chromosome 13. WES in 2 patients revealed a homozygous deletion in the promoter region of UFM1. Sanger sequencing confirmed homozygosity for this variant in all 16 patients. All patients shared a common haplotype, indicative of a founder effect. Screening of 1,000 controls from different European Roma panels demonstrated an overall carrier rate of the mutation of 3%-25%. Transfection assays showed that the deletion significantly reduced expression in specific CNS cell lines.
Conclusions: UFM1 encodes ubiquitin-fold modifier 1 (UFM1), a member of the ubiquitin-like family involved in posttranslational modification of proteins. Its exact biological role is unclear. This study associates a UFM1 gene defect with a disease and sheds new light on possible UFM1 functional networks.
Copyright © 2017 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
-
- Wolf NI, Salomons GS, Rodenburg RJ, et al. Mutations in RARS cause hypomyelination. Ann Neurol 2014;76:134–139. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
