Combined OX40L and mTOR blockade controls effector T cell activation while preserving Treg reconstitution after transplant
- PMID: 28931653
- PMCID: PMC5681253
- DOI: 10.1126/scitranslmed.aan3085
Combined OX40L and mTOR blockade controls effector T cell activation while preserving Treg reconstitution after transplant
Abstract
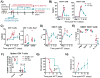
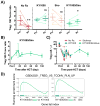
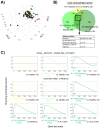
A critical question facing the field of transplantation is how to control effector T cell (Teff) activation while preserving regulatory T cell (Treg) function. Standard calcineurin inhibitor-based strategies can partially control Teffs, but breakthrough activation still occurs, and these agents are antagonistic to Treg function. Conversely, mechanistic target of rapamycin (mTOR) inhibition with sirolimus is more Treg-compatible but is inadequate to fully control Teff activation. In contrast, blockade of OX40L signaling has the capacity to partially control Teff activation despite maintaining Treg function. We used the nonhuman primate graft-versus-host disease (GVHD) model to probe the efficacy of combinatorial immunomodulation with sirolimus and the OX40L-blocking antibody KY1005. Our results demonstrate significant biologic activity of KY1005 alone (prolonging median GVHD-free survival from 8 to 19.5 days), as well as marked, synergistic control of GVHD with KY1005 + sirolimus (median survival time, >100 days; P < 0.01 compared to all other regimens), which was associated with potent control of both TH/TC1 (T helper cell 1/cytotoxic T cell 1) and TH/TC17 activation. Combined administration also maintained Treg reconstitution [resulting in an enhanced Treg/Teff ratio (40% over baseline) in the KY1005/sirolimus cohort compared to a 2.9-fold decrease in the unprophylaxed GVHD cohort]. This unique immunologic signature resulted in transplant recipients that were able to control GVHD for the length of analysis and to down-regulate donor/recipient alloreactivity despite maintaining anti-third-party responses. These data indicate that combined OX40L blockade and sirolimus represents a promising strategy to induce immune balance after transplant and is an important candidate regimen for clinical translation.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures





Comment in
-
Preserving Treg Function: Beyond mTOR Inhibitors.Transplantation. 2018 Feb;102(2):179-182. doi: 10.1097/TP.0000000000002042. Transplantation. 2018. PMID: 29319616 No abstract available.
References
-
- Peccatori J, Forcina A, Clerici D, Crocchiolo R, Vago L, Stanghellini MT, Noviello M, Messina C, Crotta A, Assanelli A, Marktel S, Olek S, Mastaglio S, Giglio F, Crucitti L, Lorusso A, Guggiari E, Lunghi F, Carrabba M, Tassara M, Battaglia M, Ferraro A, Carbone MR, Oliveira G, Roncarolo MG, Rossini S, Bernardi M, Corti C, Marcatti M, Patriarca F, Zecca M, Locatelli F, Bordignon C, Fleischhauer K, Bondanza A, Bonini C, Ciceri F. Sirolimus-based graft-versus-host disease prophylaxis promotes the in vivo expansion of regulatory T cells and permits peripheral blood stem cell transplantation from haploidentical donors. Leukemia. 2015;29:396–405. - PubMed
-
- Pidala J, Kim J, Jim H, Kharfan-Dabaja MA, Nishihori T, Fernandez HF, Tomblyn M, Perez L, Perkins J, Xu M, Janssen WE, Veerapathran A, Betts BC, Locke FL, Ayala E, Field T, Ochoa L, Alsina M, Anasetti C. A randomized phase II study to evaluate tacrolimus in combination with sirolimus or methotrexate after allogeneic hematopoietic cell transplantation. Haematologica. 2012;97:1882–1889. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

