Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs
- PMID: 28931654
- PMCID: PMC6034685
- DOI: 10.1126/scitranslmed.aan4220
Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs
Abstract
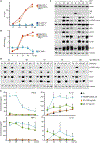
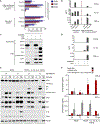
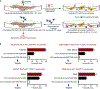
Tumors thrive in an immunosuppressive microenvironment that impedes antitumor innate and adaptive immune responses. Thus, approaches that can overcome immunosuppression and engage antitumor immunity are needed. This study defines the adjuvant and cancer immunotherapy potential of the recombinant poliovirus/rhinovirus chimera PVSRIPO. PVSRIPO is currently in clinical trials against recurrent World Health Organization grade IV malignant glioma, a notoriously treatment-refractory cancer. Cytopathogenic infection of neoplastic cells releases the proteome and exposes pathogen- and damage-associated molecular patterns. At the same time, sublethal infection of antigen-presenting cells, such as dendritic cells and macrophages, yields potent, sustained type I interferon-dominant activation in an immunosuppressed microenvironment and promotes the development of tumor antigen-specific T cell responses in vitro and antitumor immunity in vivo. PVSRIPO's immune adjuvancy stimulates canonical innate anti-pathogen inflammatory responses within the tumor microenvironment that culminate in dendritic cell and T cell infiltration. Our findings provide mechanistic evidence that PVSRIPO functions as a potent intratumor immune adjuvant that generates tumor antigen-specific cytotoxic T lymphocyte responses.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures


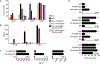


Comment in
-
Immunotherapy: When viruses attack.Nat Rev Cancer. 2017 Nov;17(11):633. doi: 10.1038/nrc.2017.97. Epub 2017 Oct 13. Nat Rev Cancer. 2017. PMID: 29026203 No abstract available.
-
Cancer immunotherapy: Targeting regulatory T cells.Nat Rev Drug Discov. 2017 Oct 30;16(11):754. doi: 10.1038/nrd.2017.206. Nat Rev Drug Discov. 2017. PMID: 29081520 No abstract available.
-
Recombinant poliovirus for cancer immunotherapy.Ann Transl Med. 2018 Sep;6(18):368. doi: 10.21037/atm.2018.07.19. Ann Transl Med. 2018. PMID: 30370295 Free PMC article. No abstract available.
References
-
- Zou W, Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 5, 263-274 (2005). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

