Pathogenic Correlates of Simian Immunodeficiency Virus-Associated B Cell Dysfunction
- PMID: 28931679
- PMCID: PMC5686751
- DOI: 10.1128/JVI.01051-17
Pathogenic Correlates of Simian Immunodeficiency Virus-Associated B Cell Dysfunction
Abstract
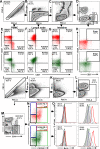
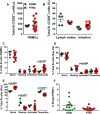
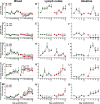
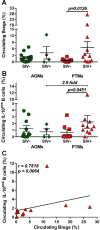
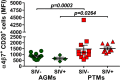
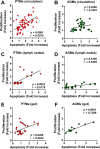
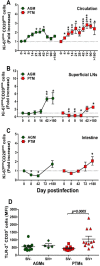
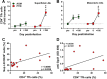
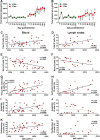
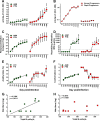
We compared and contrasted pathogenic (in pig-tailed macaques [PTMs]) and nonpathogenic (in African green monkeys [AGMs]) SIVsab infections to assess the significance of the B cell dysfunction observed in simian (SIV) and human immunodeficiency virus (HIV) infections. We report that the loss of B cells is specifically associated with the pathogenic SIV infection, while in the natural hosts, in which SIV is nonpathogenic, B cells rapidly increase in both lymph nodes (LNs) and intestine. SIV-associated B cell dysfunction associated with the pathogenic SIV infection is characterized by loss of naive B cells, loss of resting memory B cells due to their redistribution to the gut, increases of the activated B cells and circulating tissue-like memory B cells, and expansion of the B regulatory cells (Bregs). While circulating B cells are virtually restored to preinfection levels during the chronic pathogenic SIV infection, restoration is mainly due to an expansion of the "exhausted," virus-specific B cells, i.e., activated memory cells and tissue-like memory B cells. Despite of the B cell dysfunction, SIV-specific antibody (Ab) production was higher in the PTMs than in AGMs, with the caveat that rapid disease progression in PTMs was strongly associated with lack of anti-SIV Ab. Neutralization titers and the avidity and maturation of immune responses did not differ between pathogenic and nonpathogenic infections, with the exception of the conformational epitope recognition, which evolved from low to high conformations in the natural host. The patterns of humoral immune responses in the natural host are therefore more similar to those observed in HIV-infected subjects, suggesting that natural hosts may be more appropriate for modeling the immunization strategies aimed at preventing HIV disease progression. The numerous differences between the pathogenic and nonpathogenic infections with regard to dynamics of the memory B cell subsets point to their role in the pathogenesis of HIV/SIV infections and suggest that monitoring B cells may be a reliable approach for assessing disease progression.IMPORTANCE We report here that the HIV/SIV-associated B cell dysfunction (defined by loss of total and memory B cells, increased B regulatory cell [Breg] counts, and B cell activation and apoptosis) is specifically associated with pathogenic SIV infection and absent during the course of nonpathogenic SIV infection in natural nonhuman primate hosts. Alterations of the B cell population are not correlated with production of neutralizing antibodies, the levels of which are similar in the two species. Rapid progressive infections are associated with a severe impairment in SIV-specific antibody production. While we did not find major differences in avidity and maturation between the pathogenic and nonpathogenic SIV infections, we identified a major difference in conformational epitope recognition, with the nonpathogenic infection being characterized by an evolution from low to high conformations. B cell dysfunction should be considered in designing immunization strategies aimed at preventing HIV disease progression.
Keywords: B cell; follicular T helper cells; humoral immune response; immune activation; nonpathogenic infection; pathogenic infection; simian immunodeficiency virus.
Copyright © 2017 American Society for Microbiology.
Figures

Similar articles
-
Rapid Development of gp120-Focused Neutralizing B Cell Responses during Acute Simian Immunodeficiency Virus Infection of African Green Monkeys.J Virol. 2015 Sep;89(18):9485-98. doi: 10.1128/JVI.01564-15. Epub 2015 Jul 8. J Virol. 2015. PMID: 26157116 Free PMC article.
-
Critical Role for the Adenosine Pathway in Controlling Simian Immunodeficiency Virus-Related Immune Activation and Inflammation in Gut Mucosal Tissues.J Virol. 2015 Sep;89(18):9616-30. doi: 10.1128/JVI.01196-15. Epub 2015 Jul 15. J Virol. 2015. PMID: 26178986 Free PMC article.
-
Pathogenic features associated with increased virulence upon Simian immunodeficiency virus cross-species transmission from natural hosts.J Virol. 2014 Jun;88(12):6778-92. doi: 10.1128/JVI.03785-13. Epub 2014 Apr 2. J Virol. 2014. PMID: 24696477 Free PMC article.
-
The Hitchhiker Guide to CD4+ T-Cell Depletion in Lentiviral Infection. A Critical Review of the Dynamics of the CD4+ T Cells in SIV and HIV Infection.Front Immunol. 2021 Jul 21;12:695674. doi: 10.3389/fimmu.2021.695674. eCollection 2021. Front Immunol. 2021. PMID: 34367156 Free PMC article.
-
Th17 cells in natural SIV hosts.Curr Opin HIV AIDS. 2010 Mar;5(2):166-72. doi: 10.1097/COH.0b013e328335c161. Curr Opin HIV AIDS. 2010. PMID: 20543595 Review.
Cited by
-
Rapid progression is associated with lymphoid follicle dysfunction in SIV-infected infant rhesus macaques.PLoS Pathog. 2021 May 7;17(5):e1009575. doi: 10.1371/journal.ppat.1009575. eCollection 2021 May. PLoS Pathog. 2021. PMID: 33961680 Free PMC article.
-
A Pathogenic Role for Splenic B1 Cells in SIV Disease Progression in Rhesus Macaques.Front Immunol. 2019 Mar 19;10:511. doi: 10.3389/fimmu.2019.00511. eCollection 2019. Front Immunol. 2019. PMID: 30941141 Free PMC article.
-
African green monkeys avoid SIV disease progression by preventing intestinal dysfunction and maintaining mucosal barrier integrity.PLoS Pathog. 2020 Mar 2;16(3):e1008333. doi: 10.1371/journal.ppat.1008333. eCollection 2020 Mar. PLoS Pathog. 2020. PMID: 32119719 Free PMC article.
-
Regulatory T Cells As Potential Targets for HIV Cure Research.Front Immunol. 2018 Apr 13;9:734. doi: 10.3389/fimmu.2018.00734. eCollection 2018. Front Immunol. 2018. PMID: 29706961 Free PMC article. Review.
-
NK-B cell cross talk induces CXCR5 expression on natural killer cells.iScience. 2021 Sep 9;24(10):103109. doi: 10.1016/j.isci.2021.103109. eCollection 2021 Oct 22. iScience. 2021. PMID: 34622162 Free PMC article.
References
-
- Gaufin T, Pattison M, Gautam R, Stoulig C, Dufour J, MacFarland J, Mandell D, Tatum C, Marx MH, Ribeiro RM, Montefiori D, Apetrei C, Pandrea I. 2009. Effect of B-cell depletion on viral replication and clinical outcome of simian immunodeficiency virus infection in a natural host. J Virol 83:10347–10357. doi:10.1128/JVI.00880-09. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

