p21 Restricts HIV-1 in Monocyte-Derived Dendritic Cells through the Reduction of Deoxynucleoside Triphosphate Biosynthesis and Regulation of SAMHD1 Antiviral Activity
- PMID: 28931685
- PMCID: PMC5686764
- DOI: 10.1128/JVI.01324-17
p21 Restricts HIV-1 in Monocyte-Derived Dendritic Cells through the Reduction of Deoxynucleoside Triphosphate Biosynthesis and Regulation of SAMHD1 Antiviral Activity
Abstract
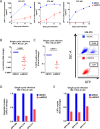
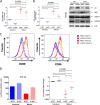
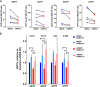
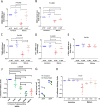
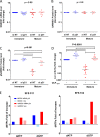
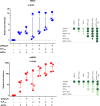
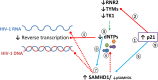
HIV-1 infection of noncycling cells, such as dendritic cells (DCs), is impaired due to limited availability of deoxynucleoside triphosphates (dNTPs), which are needed for HIV-1 reverse transcription. The levels of dNTPs are tightly regulated during the cell cycle and depend on the balance between dNTP biosynthesis and degradation. SAMHD1 potently blocks HIV-1 replication in DCs, although the underlying mechanism is still unclear. SAMHD1 has been reported to be able to degrade dNTPs and viral nucleic acids, which may both hamper HIV-1 reverse transcription. The relative contribution of these activities may differ in cycling and noncycling cells. Here, we show that inhibition of HIV-1 replication in monocyte-derived DCs (MDDCs) is associated with an increased expression of p21cip1/waf, a cell cycle regulator that is involved in the differentiation and maturation of DCs. Induction of p21 in MDDCs decreases the pool of dNTPs and increases the antiviral active isoform of SAMHD1. Although both processes are complementary in inhibiting HIV-1 replication, the antiviral activity of SAMHD1 in our primary cell model appears to be, at least partially, independent of its dNTPase activity. The reduction in the pool of dNTPs in MDDCs appears rather mostly due to a p21-mediated suppression of several enzymes involved in dNTP synthesis (i.e., RNR2, TYMS, and TK-1). These results are important to better understand the interplay between HIV-1 and DCs and may inform the design of new therapeutic approaches to decrease viral dissemination and improve immune responses against HIV-1.IMPORTANCE DCs play a key role in the induction of immune responses against HIV. However, HIV has evolved ways to exploit these cells, facilitating immune evasion and virus dissemination. We have found that the expression of p21, a cyclin-dependent kinase inhibitor involved in cell cycle regulation and monocyte differentiation and maturation, potentially can contribute to the inhibition of HIV-1 replication in monocyte-derived DCs through multiple mechanisms. p21 decreased the size of the intracellular dNTP pool. In parallel, p21 prevented SAMHD1 phosphorylation and promoted SAMHD1 dNTPase-independent antiviral activity. Thus, induction of p21 resulted in conditions that allowed the effective inhibition of HIV-1 replication through complementary mechanisms. Overall, p21 appears to be a key regulator of HIV infection in myeloid cells.
Keywords: HIV replication; HIV-1; SAMHD1; cellular factors; dNTPs; dendritic cells; p21.
Copyright © 2017 American Society for Microbiology.
Figures

References
-
- Burleigh L, Lozach PY, Schiffer C, Staropoli I, Pezo V, Porrot F, Canque B, Virelizier JL, Arenzana-Seisdedos F, Amara A. 2006. Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells. J Virol 80:2949–2957. doi: 10.1128/JVI.80.6.2949-2957.2006. - DOI - PMC - PubMed
-
- Nobile C, Petit C, Moris A, Skrabal K, Abastado JP, Mammano F, Schwartz O. 2005. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J Virol 79:5386–5399. doi: 10.1128/JVI.79.9.5386-5399.2005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

