A DNA Vaccine That Targets Hemagglutinin to Antigen-Presenting Cells Protects Mice against H7 Influenza
- PMID: 28931687
- PMCID: PMC5686743
- DOI: 10.1128/JVI.01340-17
A DNA Vaccine That Targets Hemagglutinin to Antigen-Presenting Cells Protects Mice against H7 Influenza
Abstract
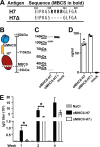
Zoonotic influenza H7 viral infections have a case fatality rate of about 40%. Currently, no or limited human to human spread has occurred, but we may be facing a severe pandemic threat if the virus acquires the ability to transmit between humans. Novel vaccines that can be rapidly produced for global distribution are urgently needed, and DNA vaccines may be the only type of vaccine that allows for the speed necessary to quench an emerging pandemic. Here, we constructed DNA vaccines encoding the hemagglutinin (HA) from influenza A/chicken/Italy/13474/99 (H7N1). In order to increase the efficacy of DNA vaccination, HA was targeted to either major histocompatibility complex class II molecules or chemokine receptors 1, 3, and 5 (CCR1/3/5) that are expressed on antigen-presenting cells (APC). A single DNA vaccination with APC-targeted HA significantly increased antibody levels in sera compared to nontargeted control vaccines. The antibodies were confirmed neutralizing in an H7 pseudotype-based neutralization assay. Furthermore, the APC-targeted vaccines increased the levels of antigen-specific cytotoxic T cells, and a single DNA vaccination could confer protection against a lethal challenge with influenza A/turkey/Italy/3889/1999 (H7N1) in mice. In conclusion, we have developed a vaccine that rapidly could contribute protection against a pandemic threat from avian influenza.IMPORTANCE Highly pathogenic avian influenza H7 constitute a pandemic threat that can cause severe illness and death in infected individuals. Vaccination is the main method of prophylaxis against influenza, but current vaccine strategies fall short in a pandemic situation due to a prolonged production time and insufficient production capabilities. In contrast, a DNA vaccine can be rapidly produced and deployed to prevent the potential escalation of a highly pathogenic influenza pandemic. We here demonstrate that a single DNA delivery of hemagglutinin from an H7 influenza could mediate full protection against a lethal challenge with H7N1 influenza in mice. Vaccine efficacy was contingent on targeting of the secreted vaccine protein to antigen-presenting cells.
Keywords: APC-targeting; DNA vaccine; avian viruses; hemagglutinin; influenza; pandemic influenza.
Copyright © 2017 Andersen et al.
Figures
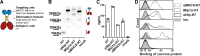




References
-
- World Health Organization. 2017. Influenza at the human-animal interface, June 2017: monthly risk assessment summary, p 1–7 World Health Organization, Geneva, Switzerland.
-
- Fouchier RaM, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SaG, Munster V, Kuiken T, Rimmelzwaan GF, Schutten M, Van Doornum GJJ, Koch G, Bosman A, Koopmans M, Osterhaus ADME. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A 101:1356–1361. doi: 10.1073/pnas.0308352100. - DOI - PMC - PubMed
-
- Zanin M, Koçer ZA, Poulson RL, Gabbard JD, Howerth EW, Jones CA, Friedman K, Seiler J, Danner A, Kercher L, McBride R, Paulson JC, Wentworth DE, Krauss S, Tompkins SM, Stallknecht DE, Webster RG. 2016. The potential for low pathogenic avian H7 influenza a viruses to replicate and cause disease in a mammalian model. J Virol 91:e01934-16. doi: 10.1128/JVI.01934-16. - DOI - PMC - PubMed
-
- Zhou L, Ren R, Yang L, Bao C, Wu J, Wang D, Li C, Xiang N, Wang Y, Li D, Sui H, Shu Y, Feng Z, Li Q, Ni D. 2017. Sudden increase in human infection with avian influenza A(H7N9) virus in China, September–December 2016. Western Pac Surveill Response J 8:6–14. doi: 10.5365/wpsar.2017.8.1.001. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

