A Selective Bottleneck Shapes the Evolutionary Mutant Spectra of Enterovirus A71 during Viral Dissemination in Humans
- PMID: 28931688
- PMCID: PMC5686718
- DOI: 10.1128/JVI.01062-17
A Selective Bottleneck Shapes the Evolutionary Mutant Spectra of Enterovirus A71 during Viral Dissemination in Humans
Abstract
RNA viruses accumulate mutations to rapidly adapt to environmental changes. Enterovirus A71 (EV-A71) causes various clinical manifestations with occasional severe neurological complications. However, the mechanism by which EV-A71 evolves within the human body is unclear. Utilizing deep sequencing and haplotype analyses of viruses from various tissues of an autopsy patient, we sought to define the evolutionary pathway by which enterovirus A71 evolves fitness for invading the central nervous system in humans. Broad mutant spectra with divergent mutations were observed at the initial infection sites in the respiratory and digestive systems. After viral invasion, we identified a haplotype switch and dominant haplotype, with glycine at VP1 residue 31 (VP1-31G) in viral particles disseminated into the integumentary and central nervous systems. In vitro viral growth and fitness analyses indicated that VP1-31G conferred growth and a fitness advantage in human neuronal cells, whereas VP1-31D conferred enhanced replication in human colorectal cells. A higher proportion of VP1-31G was also found among fatal cases, suggesting that it may facilitate central nervous system infection in humans. Our data provide the first glimpse of EV-A71 quasispecies from oral tissues to the central nervous system within humans, showing broad implications for the surveillance and pathogenesis of this reemerging viral pathogen.IMPORTANCE EV-A71 continues to be a worldwide burden to public health. Although EV-A71 is the major etiological agent of hand, foot, and mouth disease, it can also cause neurological pulmonary edema, encephalitis, and even death, especially in children. Understanding selection processes enabling dissemination and accurately estimating EV-A71 diversity during invasion in humans are critical for applications in viral pathogenesis and vaccine studies. Here, we define a selection bottleneck appearing in respiratory and digestive tissues. Glycine substitution at VP1 residue 31 helps viruses break through the bottleneck and invade the central nervous system. This substitution is also advantageous for replication in neuronal cells in vitro Considering that fatal cases contain enhanced glycine substitution at VP1-31, we suggest that the increased prevalence of VP1-31G may alter viral tropism and aid central nervous system invasion. Our findings provide new insights into a dynamic mutant spectral switch active during acute viral infection with emerging viral pathogens.
Keywords: capsid protein VP1; enterovirus A71; pathogenesis; quasispecies.
Copyright © 2017 American Society for Microbiology.
Figures




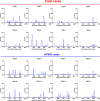
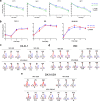



Similar articles
-
Enterovirus A71 Induces Neurological Diseases and Dynamic Variants in Oral Infection of Human SCARB2-Transgenic Weaned Mice.J Virol. 2021 Oct 13;95(21):e0089721. doi: 10.1128/JVI.00897-21. Epub 2021 Aug 11. J Virol. 2021. PMID: 34379497 Free PMC article.
-
Novel virulence determinants in VP1 regulate the assembly of enterovirus-A71.J Virol. 2024 Dec 17;98(12):e0165524. doi: 10.1128/jvi.01655-24. Epub 2024 Nov 13. J Virol. 2024. PMID: 39535185 Free PMC article.
-
Enterovirus A71 Containing Codon-Deoptimized VP1 and High-Fidelity Polymerase as Next-Generation Vaccine Candidate.J Virol. 2019 Jun 14;93(13):e02308-18. doi: 10.1128/JVI.02308-18. Print 2019 Jul 1. J Virol. 2019. PMID: 30996087 Free PMC article.
-
Viral determinants that drive Enterovirus-A71 fitness and virulence.Emerg Microbes Infect. 2021 Dec;10(1):713-724. doi: 10.1080/22221751.2021.1906754. Emerg Microbes Infect. 2021. PMID: 33745413 Free PMC article. Review.
-
Changes in the EV-A71 Genome through Recombination and Spontaneous Mutations: Impact on Virulence.Viruses. 2018 Jun 12;10(6):320. doi: 10.3390/v10060320. Viruses. 2018. PMID: 29895721 Free PMC article. Review.
Cited by
-
Humoral and cellular immunogenicity and efficacy of a coxsackievirus A10 vaccine in mice.Emerg Microbes Infect. 2023 Dec;12(1):e2147022. doi: 10.1080/22221751.2022.2147022. Emerg Microbes Infect. 2023. PMID: 36373411 Free PMC article.
-
Identification of specific and shared epitopes at the extreme N-terminal VP1 of Coxsackievirus A4, A2 and A5 by monoclonal antibodies.Virus Res. 2023 Apr 15;328:199074. doi: 10.1016/j.virusres.2023.199074. Epub 2023 Mar 1. Virus Res. 2023. PMID: 36805409 Free PMC article.
-
A VP1 mutation acquired during an enterovirus 71 disseminated infection confers heparan sulfate binding ability and modulates ex vivo tropism.PLoS Pathog. 2018 Aug 3;14(8):e1007190. doi: 10.1371/journal.ppat.1007190. eCollection 2018 Aug. PLoS Pathog. 2018. PMID: 30075025 Free PMC article.
-
Heparan sulfate attachment receptor is a major selection factor for attenuated enterovirus 71 mutants during cell culture adaptation.PLoS Pathog. 2020 Mar 18;16(3):e1008428. doi: 10.1371/journal.ppat.1008428. eCollection 2020 Mar. PLoS Pathog. 2020. PMID: 32187235 Free PMC article.
-
Electrostatic interactions at the five-fold axis alter heparin-binding phenotype and drive enterovirus A71 virulence in mice.PLoS Pathog. 2019 Nov 15;15(11):e1007863. doi: 10.1371/journal.ppat.1007863. eCollection 2019 Nov. PLoS Pathog. 2019. PMID: 31730673 Free PMC article.
References
-
- Iwai M, Masaki A, Hasegawa S, Obara M, Horimoto E, Nakamura K, Tanaka Y, Endo K, Tanaka K, Ueda J, Shiraki K, Kurata T, Takizawa T. 2009. Genetic changes of coxsackievirus A16 and enterovirus 71 isolated from hand, foot, and mouth disease patients in Toyama, Japan between 1981 and 2007. Jpn J Infect Dis 62:254–259. - PubMed
-
- Zhang Y, Wang D, Yan D, Zhu S, Liu J, Wang H, Zhao S, Yu D, Nan L, An J, Chen L, An H, Xu A, Xu W. 2010. Molecular evidence of persistent epidemic and evolution of subgenotype B1 coxsackievirus A16-associated hand, foot, and mouth disease in China. J Clin Microbiol 48:619–622. doi:10.1128/JCM.02338-09. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

