Coculture of allogenic DBM and BMSCs in the knee joint cavity of rabbits for cartilage tissue engineering
- PMID: 28931727
- PMCID: PMC5968190
- DOI: 10.1042/BSR20170804
Coculture of allogenic DBM and BMSCs in the knee joint cavity of rabbits for cartilage tissue engineering
Abstract
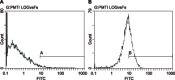
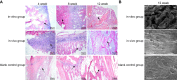
The present study aims to assess coculture of allogenic decalcified bone matrix (DBM) and bone marrow mesenchymal stem cells (BMSCs) in the knee joint cavity of rabbits for cartilage tissue engineering. Rabbits were assigned to an in vitro group, an in vivo group, and a blank control group. At the 4th, 8th, and 12th week, samples from all groups were collected for hematoxylin-eosin (HE) staining and streptavidin-peroxidase (SP) method. The morphological analysis software was used to calculate the average absorbance value (A value). SP and flow cytometry demonstrated that BMSCs were induced into chondrocytes. DBM scaffold showed honeycomb-shaped porous and three-dimensional structure, while the surface pores are interlinked with the deep pores. At the 4th week, in the blank control group, DBM scaffold structure was clear, and cells analogous to chondrocytes were scattered in the interior of DBM scaffolds. At the 8th week, in the in vivo group, there were a large amount of cells, mainly mature chondrocytes, and the DBM scaffolds were partially absorbed. At the 12th week, in the in vitro group, the interior of scaffolds was filled up with chondrocytes with partial fibrosis, but arranged in disorder. In the in vivo group, the chondrocytes completely infiltrated into the interior of scaffolds and were arranged in certain stress direction. The in vivo group showed higher A value than the in vitro and blank control groups at each time point. Allogenic DBM combined BMSCs in the knee joint cavity of rabbits could provide better tissue-engineered cartilage than that cultivated in vitro.
Keywords: Bone marrow derived mesenchymal stem cells; Cartilage tissue engineering; Decalcified bone matrix; Knee joint cavity; Rabbit.
© 2017 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



Similar articles
-
Transplantation of allogenic chondrocytes with chitosan hydrogel-demineralized bone matrix hybrid scaffold to repair rabbit cartilage injury.Biomaterials. 2016 Nov;108:157-67. doi: 10.1016/j.biomaterials.2016.09.002. Epub 2016 Sep 6. Biomaterials. 2016. PMID: 27636153
-
Experimental study on allogenic decalcified bone matrix as carrier for bone tissue engineering.J Huazhong Univ Sci Technolog Med Sci. 2004;24(2):147-50. doi: 10.1007/BF02885415. J Huazhong Univ Sci Technolog Med Sci. 2004. PMID: 15315166
-
[Effect of bone marrow mesenchymal stem cells-derived extracellular matrix scaffold on chondrogenic differentiation of marrow clot after microfracture of bone marrow stimulation in vitro].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013 Apr;27(4):464-74. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013. PMID: 23757877 Chinese.
-
[Recent progress of BMSCs acting as seeding cell for tissue engineered cartilage].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008 Feb;22(2):163-6. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008. PMID: 18365611 Review. Chinese.
-
[Progress of methods of inducing bone marrow mesenchymal stem cells into chondrocytes in vitro].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 May;25(5):618-23. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011. PMID: 21675125 Review. Chinese.
Cited by
-
3D Cartilage Regeneration With Certain Shape and Mechanical Strength Based on Engineered Cartilage Gel and Decalcified Bone Matrix.Front Cell Dev Biol. 2021 Feb 26;9:638115. doi: 10.3389/fcell.2021.638115. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33718376 Free PMC article.
-
Construction of three-dimensional, homogeneous regenerative cartilage tissue based on the ECG-DBM complex.Front Bioeng Biotechnol. 2023 Sep 25;11:1252790. doi: 10.3389/fbioe.2023.1252790. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37818235 Free PMC article.
References
-
- Wu J., Wu D., Guo K., Yuan F. and Ran B. (2014) OPN polymorphism is associated with the susceptibility to cervical spondylotic myelopathy and its outcome after anterior cervical corpectomy and fusion. Cell. Physiol. Biochem. 34, 565–574 - PubMed
-
- Abode-Iyamah K.O., Stoner K.E., Grossbach A.J., Viljoen S.V., McHenry C.L., Petrie M.A. et al. (2016) Effects of brain derived neurotrophic factor Val66Met polymorphism in patients with cervical spondylotic myelopathy. J. Clin. Neurosci. 24, 117–121 - PubMed
-
- Lazebnik M., Singh M., Glatt P., Friis L.A., Berkland C.J. and Detamore M.S. (2011) Biomimetic method for combining the nucleus pulposus and annulus fibrosus for intervertebral disc tissue engineering. J. Tissue Eng. Regen. Med. 5, e179–187 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

