Combination central tolerance and peripheral checkpoint blockade unleashes antimelanoma immunity
- PMID: 28931755
- PMCID: PMC5621898
- DOI: 10.1172/jci.insight.93265
Combination central tolerance and peripheral checkpoint blockade unleashes antimelanoma immunity
Abstract
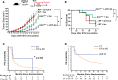
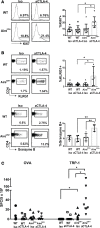
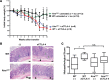
Blockade of immune checkpoint proteins (e.g., CTLA-4, PD-1) improves overall survival in advanced melanoma; however, therapeutic benefit is limited to only a subset of patients. Because checkpoint blockade acts by "removing the brakes" on effector T cells, the efficacy of checkpoint blockade may be constrained by the limited pool of melanoma-reactive T cells in the periphery. In the thymus, autoimmune regulator (Aire) promotes deletion of T cells reactive against self-antigens that are also expressed by tumors. Thus, while protecting against autoimmunity, Aire also limits the generation of melanoma-reactive T cells. Here, we show that Aire deficiency in mice expands the pool of CD4+ T cells capable of melanoma cell eradication and has additive effects with anti-CTLA-4 antibody in slowing melanoma tumor growth and increasing survival. Moreover, pharmacologic blockade of central T cell tolerance and peripheral checkpoint blockade in combination enhanced antimelanoma immunity in a synergistic manner. In melanoma patients treated with anti-CTLA-4 antibody, clinical response to therapy was associated with a human Aire polymorphism. Together, these findings suggest that Aire-mediated central tolerance constrains the efficacy of peripheral checkpoint inhibition and point to simultaneous blockade of Aire and checkpoint inhibitors as a novel strategy to enhance antimelanoma immunity.
Keywords: Cancer immunotherapy; Cellular immune response; Immunology; Melanoma.
Conflict of interest statement
Figures



Similar articles
-
Ctla-4 blockade plus adoptive T-cell transfer promotes optimal melanoma immunity in mice.J Immunother. 2015 Feb-Mar;38(2):54-61. doi: 10.1097/CJI.0000000000000064. J Immunother. 2015. PMID: 25658614 Free PMC article.
-
Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade.Cell. 2017 Sep 7;170(6):1120-1133.e17. doi: 10.1016/j.cell.2017.07.024. Epub 2017 Aug 10. Cell. 2017. PMID: 28803728 Free PMC article.
-
Checkpoint blockade immunotherapy enhances the frequency and effector function of murine tumor-infiltrating T cells but does not alter TCRβ diversity.Cancer Immunol Immunother. 2019 Jul;68(7):1095-1106. doi: 10.1007/s00262-019-02346-4. Epub 2019 May 18. Cancer Immunol Immunother. 2019. PMID: 31104075 Free PMC article.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients.Clin Cancer Res. 2013 Oct 1;19(19):5300-9. doi: 10.1158/1078-0432.CCR-13-0143. Clin Cancer Res. 2013. PMID: 24089443 Review.
Cited by
-
Single-cell multiomics defines tolerogenic extrathymic Aire-expressing populations with unique homology to thymic epithelium.Sci Immunol. 2021 Nov 12;6(65):eabl5053. doi: 10.1126/sciimmunol.abl5053. Epub 2021 Nov 12. Sci Immunol. 2021. PMID: 34767455 Free PMC article.
-
PD-1 cooperates with AIRE-mediated tolerance to prevent lethal autoimmune disease.Proc Natl Acad Sci U S A. 2022 Apr 12;119(15):e2120149119. doi: 10.1073/pnas.2120149119. Epub 2022 Apr 8. Proc Natl Acad Sci U S A. 2022. PMID: 35394861 Free PMC article.
-
Thymic tolerance as a key brake on autoimmunity.Nat Immunol. 2018 Jul;19(7):659-664. doi: 10.1038/s41590-018-0128-9. Epub 2018 Jun 20. Nat Immunol. 2018. PMID: 29925986 Free PMC article. Review.
-
A peripheral immune signature of responsiveness to PD-1 blockade in patients with classical Hodgkin lymphoma.Nat Med. 2020 Sep;26(9):1468-1479. doi: 10.1038/s41591-020-1006-1. Epub 2020 Aug 10. Nat Med. 2020. PMID: 32778827 Free PMC article. Clinical Trial.
-
Immune Modulation with RANKL Blockade through Denosumab Treatment in Patients with Cancer.Cancer Immunol Res. 2024 Apr 2;12(4):453-461. doi: 10.1158/2326-6066.CIR-23-0184. Cancer Immunol Res. 2024. PMID: 38276989 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

