Monocyte dysregulation and systemic inflammation during pediatric falciparum malaria
- PMID: 28931756
- PMCID: PMC5621919
- DOI: 10.1172/jci.insight.95352
Monocyte dysregulation and systemic inflammation during pediatric falciparum malaria
Abstract
Background: Inflammation and monocytes are thought to be important to human malaria pathogenesis. However, the relationship of inflammation and various monocyte functions to acute malaria, recovery from acute malaria, and asymptomatic parasitemia in endemic populations is poorly understood.
Methods: We evaluated plasma cytokine levels, monocyte subsets, monocyte functional responses, and monocyte inflammatory transcriptional profiles of 1- to 10-year-old Kenyan children at the time of presentation with acute uncomplicated malaria and at recovery 6 weeks later; these results were compared with analogous data from asymptomatic children and adults in the same community.
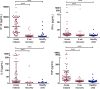
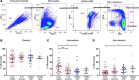
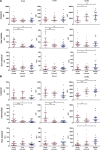
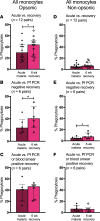
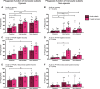
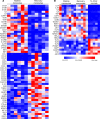
Results: Acute malaria was marked by elevated levels of proinflammatory and regulatory cytokines and expansion of the inflammatory "intermediate" monocyte subset that returned to levels of healthy asymptomatic children 6 weeks later. Monocytes displayed activated phenotypes during acute malaria, with changes in surface expression of markers important to innate and adaptive immunity. Functionally, acute malaria monocytes and monocytes from asymptomatic infected children had impaired phagocytosis of P. falciparum-infected erythrocytes relative to asymptomatic children with no blood-stage infection. Monocytes from both acute malaria and recovery time points displayed strong and equivalent cytokine responsiveness to innate immune agonists that were independent of infection status. Monocyte transcriptional profiles revealed regulated and balanced proinflammatory and antiinflammatory and altered phagocytosis gene expression patterns distinct from malaria-naive monocytes.
Conclusion: These observations provide insights into monocyte functions and the innate immune response during uncomplicated malaria and suggest that asymptomatic parasitemia in children is not clinically benign.
Funding: Support for this work was provided by NIH/National Institute of Allergy and Infectious Diseases (R01AI095192-05), the Burroughs Wellcome Fund/American Society of Tropical Medicine and Hygiene, and the Rainbow Babies & Children's Foundation.
Keywords: Immunology; Infectious disease; Innate immunity; Malaria; Monocytes.
Conflict of interest statement
Figures


Similar articles
-
Plasmodium falciparum malaria drives epigenetic reprogramming of human monocytes toward a regulatory phenotype.PLoS Pathog. 2021 Apr 6;17(4):e1009430. doi: 10.1371/journal.ppat.1009430. eCollection 2021 Apr. PLoS Pathog. 2021. PMID: 33822828 Free PMC article.
-
Soluble markers of neutrophil, T-cell and monocyte activation are associated with disease severity and parasitemia in falciparum malaria.BMC Infect Dis. 2018 Dec 18;18(1):670. doi: 10.1186/s12879-018-3593-8. BMC Infect Dis. 2018. PMID: 30563486 Free PMC article.
-
Exposure-dependent control of malaria-induced inflammation in children.PLoS Pathog. 2014 Apr 17;10(4):e1004079. doi: 10.1371/journal.ppat.1004079. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24743880 Free PMC article. Clinical Trial.
-
Malaria blood-stage infection and its control by the immune system.Folia Biol (Praha). 2000;46(6):210-8. Folia Biol (Praha). 2000. PMID: 11140853 Review.
-
The Rough Guide to Monocytes in Malaria Infection.Front Immunol. 2018 Dec 7;9:2888. doi: 10.3389/fimmu.2018.02888. eCollection 2018. Front Immunol. 2018. PMID: 30581439 Free PMC article. Review.
Cited by
-
Transcriptional profiling and immunophenotyping show sustained activation of blood monocytes in subpatent Plasmodium falciparum infection.Clin Transl Immunology. 2020 Jun 18;9(6):e1144. doi: 10.1002/cti2.1144. eCollection 2020. Clin Transl Immunology. 2020. PMID: 32566226 Free PMC article.
-
Changes in the Molecular and Functional Phenotype of Bovine Monocytes during Theileria parva Infection.Infect Immun. 2019 Nov 18;87(12):e00703-19. doi: 10.1128/IAI.00703-19. Print 2019 Dec. Infect Immun. 2019. PMID: 31570561 Free PMC article.
-
Activation of cGAS-STING by Lethal Malaria N67C Dictates Immunity and Mortality through Induction of CD11b+ Ly6Chi Proinflammatory Monocytes.Adv Sci (Weinh). 2022 Aug;9(22):e2103701. doi: 10.1002/advs.202103701. Epub 2022 May 29. Adv Sci (Weinh). 2022. PMID: 35635376 Free PMC article.
-
Lymphocyte crosstalk is required for monocyte-intrinsic trained immunity to Plasmodium falciparum.J Clin Invest. 2022 Jun 1;132(11):e139298. doi: 10.1172/JCI139298. J Clin Invest. 2022. PMID: 35642634 Free PMC article.
-
Integrative analysis of microRNA and mRNA expression profiles of monocyte-derived dendritic cells differentiation during experimental cerebral malaria.J Leukoc Biol. 2020 Oct;108(4):1183-1197. doi: 10.1002/JLB.1MA0320-731R. Epub 2020 May 3. J Leukoc Biol. 2020. PMID: 32362022 Free PMC article.
References
-
- World Malaria Report 2015. World Health Organization. http://www.who.int/malaria/publications/world-malaria-report-2015/report... Accessed August 24, 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

