Reversible retinal vessel closure from VEGF-induced leukocyte plugging
- PMID: 28931763
- PMCID: PMC5621911
- DOI: 10.1172/jci.insight.95530
Reversible retinal vessel closure from VEGF-induced leukocyte plugging
Abstract
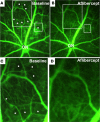
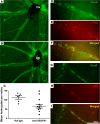
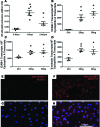
Clinical trials in patients with macular edema due to diabetic retinopathy or retinal vein occlusion (RVO) have shown that suppression of VEGF not only improves macular edema, but also reopens closed retinal vessels, prevents progression of vessel closure, and improves retinopathy. In this study, we show the molecular basis for those clinical observations. Increased retinal levels of VEGF in mice cause plugging of retinal vessels with leukocytes, vessel closure, and hypoxia. Suppression of VEGF reduces leukocyte plugging, causing reperfusion of closed vessels. Activation of VEGFR1 contributes to leukocyte recruitment, because it is significantly reduced by an anti-VEGFR1-neutralizing antibody. High VEGF increases transcriptional activity of NF-κB and expression of NF-κB target genes, particularly Vcam1. Injection of an anti-VCAM-1-neutralizing antibody reduces VEGF-induced leukocyte plugging. These data explain the broad range of benefits obtained by VEGF suppression in patients with ischemic retinopathies, provide an important insight into the pathogenesis of RVO and diabetic retinopathy, and suggest that sustained suppression of VEGF early in the course of these diseases may prevent vessel closure, worsening ischemia, and disease progression. This study also identifies VEGFR1 and VCAM-1 as molecular targets whose suppression could supplement VEGF neutralization for treatment of RVO and diabetic retinopathy.
Keywords: Retinopathy; Vascular Biology; endothelial cells; hypoxia.
Conflict of interest statement
Figures
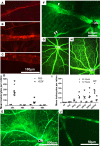
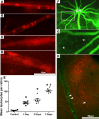
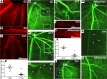
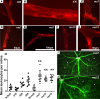

References
-
- [No authors listed] Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. doi: 10.1016/S0140-6736(98)07019-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

