Automatic and adaptive heterogeneous refractive index compensation for light-sheet microscopy
- PMID: 28931809
- PMCID: PMC5606987
- DOI: 10.1038/s41467-017-00514-7
Automatic and adaptive heterogeneous refractive index compensation for light-sheet microscopy
Abstract
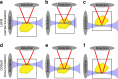
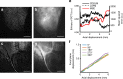
Optical tissue clearing has revolutionized researchers' ability to perform fluorescent measurements of molecules, cells, and structures within intact tissue. One common complication to all optically cleared tissue is a spatially heterogeneous refractive index, leading to light scattering and first-order defocus. We designed C-DSLM (cleared tissue digital scanned light-sheet microscopy) as a low-cost method intended to automatically generate in-focus images of cleared tissue. We demonstrate the flexibility and power of C-DSLM by quantifying fluorescent features in tissue from multiple animal models using refractive index matched and mismatched microscope objectives. This includes a unique measurement of myelin tracks within intact tissue using an endogenous fluorescent reporter where typical clearing approaches render such structures difficult to image. For all measurements, we provide independent verification using standard serial tissue sectioning and quantification methods. Paired with advancements in volumetric image processing, C-DSLM provides a robust methodology to quantify sub-micron features within large tissue sections.Optical clearing of tissue has enabled optical imaging deeper into tissue due to significantly reduced light scattering. Here, Ryan et al. tackle first-order defocus, an artefact of a non-uniform refractive index, extending light-sheet microscopy to partially cleared samples.
Conflict of interest statement
The University of Colorado has filed a patent for parts of this work. D.P.R. and D.P.S. are the co-inventors and the patent is assigned to D.P.R. and the University of Colorado. The remaining authors declare no competing finanical interests.
Figures




References
-
- Siedentopf H, Zsigmondy R. Uber sichtbarmachung und größenbestimmung ultramikoskopischer teilchen, mit besonderer anwendung auf goldrubingläser. Ann. Phys. 1902;315:1–39. doi: 10.1002/andp.19023150102. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

