TGFβR signalling controls CD103+CD11b+ dendritic cell development in the intestine
- PMID: 28931816
- PMCID: PMC5607002
- DOI: 10.1038/s41467-017-00658-6
TGFβR signalling controls CD103+CD11b+ dendritic cell development in the intestine
Abstract
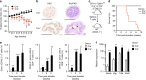
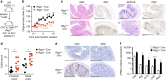
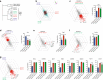
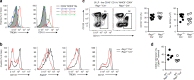
CD103+CD11b+ dendritic cells (DCs) are unique to the intestine, but the factors governing their differentiation are unclear. Here we show that transforming growth factor receptor 1 (TGFβR1) has an indispensable, cell intrinsic role in the development of these cells. Deletion of Tgfbr1 results in markedly fewer intestinal CD103+CD11b+ DCs and a reciprocal increase in the CD103-CD11b+ dendritic cell subset. Transcriptional profiling identifies markers that define the CD103+CD11b+ DC lineage, including CD101, TREM1 and Siglec-F, and shows that the absence of CD103+CD11b+ DCs in CD11c-Cre.Tgfbr1 fl/fl mice reflects defective differentiation from CD103-CD11b+ intermediaries, rather than an isolated loss of CD103 expression. The defect in CD103+CD11b+ DCs is accompanied by reduced generation of antigen-specific, inducible FoxP3+ regulatory T cells in vitro and in vivo, and by reduced numbers of endogenous Th17 cells in the intestinal mucosa. Thus, TGFβR1-mediated signalling may explain the tissue-specific development of these unique DCs.Developmental cues for the different dendritic cell (DC) subsets in the intestine are yet to be defined. Here the authors show that TGFβR1 signalling is needed for development of CD103+CD11b+ intestinal DCs from CD103-CD11b+ cells and that they contribute to the generation of Th17 and regulatory T cells.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. 2003;3:331–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

