Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response
- PMID: 28931823
- PMCID: PMC5606984
- DOI: 10.1038/s41467-017-00729-8
Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response
Abstract
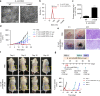
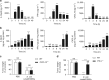
Gram-negative bacteria actively secrete outer membrane vesicles, spherical nano-meter-sized proteolipids enriched with outer membrane proteins, to the surroundings. Outer membrane vesicles have gained wide interests as non-living complex vaccines or delivery vehicles. However, no study has used outer membrane vesicles in treating cancer thus far. Here we investigate the potential of bacterial outer membrane vesicles as therapeutic agents to treat cancer via immunotherapy. Our results show remarkable capability of bacterial outer membrane vesicles to effectively induce long-term antitumor immune responses that can fully eradicate established tumors without notable adverse effects. Moreover, systematically administered bacterial outer membrane vesicles specifically target and accumulate in the tumor tissue, and subsequently induce the production of antitumor cytokines CXCL10 and interferon-γ. This antitumor effect is interferon-γ dependent, as interferon-γ-deficient mice could not induce such outer membrane vesicle-mediated immune response. Together, our results herein demonstrate the potential of bacterial outer membrane vesicles as effective immunotherapeutic agent that can treat various cancers without apparent adverse effects.Bacterial outer membrane vesicles (OMVs) contain immunogens but no study has yet examined their potential in treating cancer. Here, the authors demonstrate that OMVs can suppress established tumours and prevent tumour metastasis by an interferon-γ mediated antitumor response.
Conflict of interest statement
O.Y.K. and Y.S.G. are co-owners of patent entitled ‘Method for treating and diagnosing cancer by using cell-derived microvesicles’ patent number: WO2012002760 A2. The remaining authors declare no competing financial interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

