Hyperhomocysteinemia Alters Retinal Endothelial Cells Barrier Function and Angiogenic Potential via Activation of Oxidative Stress
- PMID: 28931831
- PMCID: PMC5607263
- DOI: 10.1038/s41598-017-09731-y
Hyperhomocysteinemia Alters Retinal Endothelial Cells Barrier Function and Angiogenic Potential via Activation of Oxidative Stress
Abstract
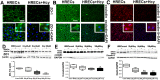
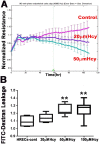
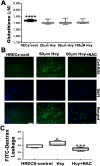
Hyperhomocysteinemia (HHcy) is associated with several human visual disorders, such as diabetic retinopathy (DR) and age-related macular degeneration (AMD). Breakdown of the blood-retinal barrier (BRB) is linked to vision loss in DR and AMD. Our previous work revealed that HHcy altered BRB in retinal endothelial cells in vivo. Here we hypothesize that homocysteine (Hcy) alters retinal endothelial cell barrier function and angiogenic potential via activation of oxidative stress. Human retinal endothelial cells (HRECs) treated with and without different concentrations of Hcy showed a reduction of tight junction protein expression, increased FITC dextran leakage, decreased transcellular electrical resistance and increased angiogenic potential. In addition, HRECs treated with Hcy showed increased production of reactive oxygen species (ROS). The anti-oxidant N-acetyl-cysteine (NAC) reduced ROS formation and decreased FITC-dextran leakage in Hcy treated HRECs. A mouse model of HHcy, in which cystathionine-β-synthase is deficient (cbs -/-), was evaluated for oxidative stress by dichlolorofluorescein (DCF), dihydroethidium (DHE) staining. There was a marked increase in ROS production and augmented GSH reductase and antioxidant regulator NRF2 activity, but decreased antioxidant gene expression in retinas of hyperhomocysteinemic mice. Our results suggest activation of oxidative stress as a possible mechanism of HHcy induced retinal endothelial cell dysfunction.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Implication of N-Methyl-d-Aspartate Receptor in Homocysteine-Induced Age-Related Macular Degeneration.Int J Mol Sci. 2021 Aug 28;22(17):9356. doi: 10.3390/ijms22179356. Int J Mol Sci. 2021. PMID: 34502266 Free PMC article.
-
N-Methyl-D-aspartate receptor activation, novel mechanism of homocysteine-induced blood-retinal barrier dysfunction.J Mol Med (Berl). 2021 Jan;99(1):119-130. doi: 10.1007/s00109-020-02000-y. Epub 2020 Nov 6. J Mol Med (Berl). 2021. PMID: 33159240 Free PMC article.
-
Alterations of retinal vasculature in cystathionine-Beta-synthase mutant mice, a model of hyperhomocysteinemia.Invest Ophthalmol Vis Sci. 2013 Feb 1;54(2):939-49. doi: 10.1167/iovs.12-10536. Invest Ophthalmol Vis Sci. 2013. PMID: 23307965 Free PMC article.
-
Implication of Hyperhomocysteinemia in Blood Retinal Barrier (BRB) Dysfunction.Biomolecules. 2020 Jul 29;10(8):1119. doi: 10.3390/biom10081119. Biomolecules. 2020. PMID: 32751132 Free PMC article. Review.
-
Mechanisms of cardiovascular remodeling in hyperhomocysteinemia.Antioxid Redox Signal. 2011 Oct 1;15(7):1927-43. doi: 10.1089/ars.2010.3721. Epub 2011 Apr 21. Antioxid Redox Signal. 2011. PMID: 21126196 Free PMC article. Review.
Cited by
-
Homocysteine Disrupts Balance between MMP-9 and Its Tissue Inhibitor in Diabetic Retinopathy: The Role of DNA Methylation.Int J Mol Sci. 2020 Mar 5;21(5):1771. doi: 10.3390/ijms21051771. Int J Mol Sci. 2020. PMID: 32150828 Free PMC article.
-
Implication of N-Methyl-d-Aspartate Receptor in Homocysteine-Induced Age-Related Macular Degeneration.Int J Mol Sci. 2021 Aug 28;22(17):9356. doi: 10.3390/ijms22179356. Int J Mol Sci. 2021. PMID: 34502266 Free PMC article.
-
Hyperhomocysteinemia-Driven Ischemic Stroke: Unraveling Molecular Mechanisms and Therapeutic Horizons.Food Sci Nutr. 2025 Jul 3;13(7):e70517. doi: 10.1002/fsn3.70517. eCollection 2025 Jul. Food Sci Nutr. 2025. PMID: 40612136 Free PMC article. Review.
-
Homocysteine and Age-Related Central Nervous System Diseases: Role of Inflammation.Int J Mol Sci. 2021 Jun 10;22(12):6259. doi: 10.3390/ijms22126259. Int J Mol Sci. 2021. PMID: 34200792 Free PMC article. Review.
-
Exploring the role of Müller cells-derived exosomes in diabetic retinopathy.Microvasc Res. 2024 Jul;154:104695. doi: 10.1016/j.mvr.2024.104695. Epub 2024 May 8. Microvasc Res. 2024. PMID: 38723843 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

