Complications following vaginal mesh procedures for stress urinary incontinence: an 8 year study of 92,246 women
- PMID: 28931856
- PMCID: PMC5607307
- DOI: 10.1038/s41598-017-11821-w
Complications following vaginal mesh procedures for stress urinary incontinence: an 8 year study of 92,246 women
Abstract
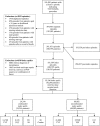
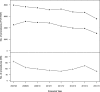
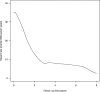
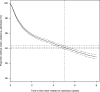
Complications of surgical mesh procedures have led to legal cases against manufacturers worldwide and to national inquiries about their safety. The aim of this study was to investigate the rate of adverse events of these procedures for stress urinary incontinence in England over 8 years. This was a retrospective cohort study of first-time tension-free vaginal tape (TVT), trans-obturator tape (TOT) or suprapubic sling (SS) surgical mesh procedures between April 2007 and March 2015. Cases were identified from the Hospital Episode Statistics database. Outcomes included number and type of procedures, including those potentially confounded by concomitant procedures, and frequency, nature and timing of complications. 92,246 first-time surgical mesh procedures (56,648 TVT, 34,704 TOT, 834 SS and 60 combinations) were identified, including 68,002 unconfounded procedures. Peri-procedural and 30-day complication rates in the unconfounded cohort were 2.4 [2.3-2.5]% and 1.7 [1.6-1.8]% respectively; 5.9 [5.7-6.1]% were readmitted at least once within 5 years for further mesh intervention or symptoms of complications, the highest risk being within the first 2 years. Complication rates were higher in the potentially confounded cohort. The complication rate within 5 years of the mesh procedure was 9.8 [9.6:10.0]% This evidence can inform future decision-making on this procedure.
Conflict of interest statement
A.M. declares personal fees from Pfizer, personal fees from Astellas, personal fees from SEP Pharma, outside the submitted work. All remaining authors (K.K., S.E., H.P., J.P., B.C., A.J.S.) declare no competing financial interests.
Figures
References
-
- Medicines and Healthcare Products Regulatory Agency, MHRA. A summary of the evidence on the benefits and risks of vaginal mesh implants. https://www.gov.uk/government/publications/vaginal-mesh-implants-summary... (2014).
-
- U.S. Food and Drug Administration, FDA. U.S. Food and Drug Administration Executive Summary: Reclassification of Urogynecologic Surgical Mesh Instrumentation. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMateria... (2016).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

