Design, synthesis and structure-activity relationship of 3,6-diaryl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines as novel tubulin inhibitors
- PMID: 28931885
- PMCID: PMC5607265
- DOI: 10.1038/s41598-017-10860-7
Design, synthesis and structure-activity relationship of 3,6-diaryl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines as novel tubulin inhibitors
Abstract
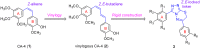
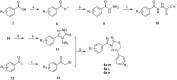
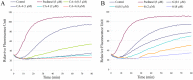
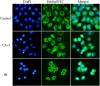
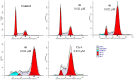
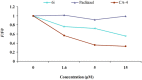
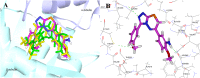
A novel series of 3,6-diaryl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines were designed, synthesized and biologically evaluated as vinylogous CA-4 analogues, which involved a rigid [1,2,4]triazolo[3,4-b][1,3,4]thiadiazine scaffold to fix the configuration of (Z,E)-butadiene linker of A-ring and B-ring. Among these rigidly vinylogous CA-4 analogues, compounds 4d, 5b, 5i, 6c, 6e, 6g, 6i and 6k showed excellent antiproliferative activities against SGC-7901, A549 and HT-1080 cell lines with IC50 values at the nanomolar level. Compound 6i showed the most highly active antiproliferative activity against the three human cancer cell lines with an IC50 values of 0.011-0.015 µM, which are comparable to those of CA-4 (IC50 = 0.009-0.013 µM). Interestingly, SAR studies revealed that 3,4-methylenedioxyphenyl, 3,4-dimethoxyphenyl, 3-methoxyphenyl and 4-methoxyphenyl could replace the classic 3,4,5-trimethoxyphenyl in CA-4 structure and keep antiproliferative activity in this series of designed compounds. Tubulin polymerization experiments showed that 6i could effectively inhibit tubulin polymerization, which was corresponded with CA-4, and immunostaining experiments suggested that 6i significantly disrupted microtubule/tubulin dynamics. Furthermore, 6i potently induced cell cycle arrest at G2/M phase in SGC-7901 cells. Competitive binding assays and docking studies suggested that compound 6i binds to the tubulin perfectly at the colchicine binding site. Taken together, these results revealed that 6i may become a promising lead compound for new anticancer drugs discovery.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Design and discovery of new antiproliferative 1,2,4-triazin-3(2H)-ones as tubulin polymerization inhibitors targeting colchicine binding site.Bioorg Chem. 2021 Jul;112:104965. doi: 10.1016/j.bioorg.2021.104965. Epub 2021 May 5. Bioorg Chem. 2021. PMID: 34020238
-
Design, synthesis and bioevaluation of 2,7-diaryl-pyrazolo[1,5-a]pyrimidines as tubulin polymerization inhibitors.Bioorg Chem. 2021 Oct;115:105220. doi: 10.1016/j.bioorg.2021.105220. Epub 2021 Jul 29. Bioorg Chem. 2021. PMID: 34352709
-
Design, synthesis and bioevaluation of antitubulin agents carrying diaryl-5,5-fused-heterocycle scaffold.Eur J Med Chem. 2017 Oct 20;139:242-249. doi: 10.1016/j.ejmech.2017.05.065. Epub 2017 Jun 1. Eur J Med Chem. 2017. PMID: 28802124
-
Combretastatin A-4 based compounds as potential anticancer agents: A review.Bioorg Chem. 2024 Dec;153:107930. doi: 10.1016/j.bioorg.2024.107930. Epub 2024 Oct 29. Bioorg Chem. 2024. PMID: 39504638 Review.
-
QSAR and 3D-QSAR models in the field of tubulin inhibitors as anticancer agents.Curr Top Med Chem. 2014;14(20):2253-62. doi: 10.2174/1568026614666141130092853. Curr Top Med Chem. 2014. PMID: 25434357 Review.
Cited by
-
Vision on Synthetic and Medicinal Facets of 1,2,4-Triazolo[3,4-b][1,3,4]thiadiazine Scaffold.Top Curr Chem (Cham). 2022 Feb 5;380(2):10. doi: 10.1007/s41061-022-00365-x. Top Curr Chem (Cham). 2022. PMID: 35122161 Free PMC article. Review.
-
Antitumor evaluation of novel phenothiazine derivatives that inhibit migration and tubulin polymerization against gastric cancer MGC-803 cells.Invest New Drugs. 2019 Feb;37(1):188-198. doi: 10.1007/s10637-018-0682-x. Epub 2018 Oct 22. Invest New Drugs. 2019. PMID: 30345465
-
Convenient synthesis and X-ray determination of 2-amino-6H-1,3,4-thiadiazin-3-ium bromides endowed with antiproliferative activity.RSC Adv. 2024 Jun 27;14(25):17866-17876. doi: 10.1039/d4ra02531h. eCollection 2024 May 28. RSC Adv. 2024. PMID: 38939040 Free PMC article.
-
3,4-Diarylisoxazoles-Analogues of Combretastatin A-4: Design, Synthesis, and Biological Evaluation In Vitro and In Vivo.ACS Pharmacol Transl Sci. 2024 Jan 16;7(2):384-394. doi: 10.1021/acsptsci.3c00239. eCollection 2024 Feb 9. ACS Pharmacol Transl Sci. 2024. PMID: 38357282 Free PMC article.
-
Synthetic Methods and Pharmacological Potentials of Triazolothiadiazines: A Review.Molecules. 2024 Mar 16;29(6):1326. doi: 10.3390/molecules29061326. Molecules. 2024. PMID: 38542962 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

