Pathogenesis of Lethal Aspiration Pneumonia in Mecp2-null Mouse Model for Rett Syndrome
- PMID: 28931890
- PMCID: PMC5607245
- DOI: 10.1038/s41598-017-12293-8
Pathogenesis of Lethal Aspiration Pneumonia in Mecp2-null Mouse Model for Rett Syndrome
Abstract
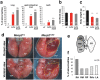
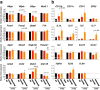
Rett syndrome (RTT) is a neurodevelopmental disorder mainly caused by mutations in the gene encoding the transcriptional regulator Methyl-CpG-binding protein 2 (MeCP2), located on the X chromosome. Many RTT patients have breathing abnormalities, such as apnea and breathing irregularity, and respiratory infection is the most common cause of death in these individuals. Previous studies showed that MeCP2 is highly expressed in the lung, but its role in pulmonary function remains unknown. In this study, we found that MeCP2 deficiency affects pulmonary gene expression and structures. We also found that Mecp2-null mice, which also have breathing problems, often exhibit inflammatory lung injury. These injuries occurred in specific sites in the lung lobes. In addition, polarizable foreign materials were identified in the injured lungs of Mecp2-null mice. These results indicated that aspiration might be a cause of inflammatory lung injury in Mecp2-null mice. On the other hand, MeCP2 deficiency affected the expression of several neuromodulator genes in the lower brainstem. Among them, neuropeptide substance P (SP) immunostaining was reduced in Mecp2-null brainstem. These findings suggest that alteration of SP expression in brainstem may be involved in autonomic dysregulation, and may be one of the causes of aspiration in Mecp2-null mice.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

