Plasmonic Enhancement of Selective Photonic Virus Inactivation
- PMID: 28931903
- PMCID: PMC5607298
- DOI: 10.1038/s41598-017-12377-5
Plasmonic Enhancement of Selective Photonic Virus Inactivation
Abstract
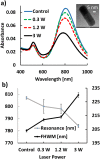
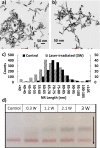
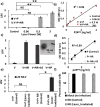
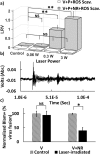
Femtosecond (fs) pulsed laser irradiation techniques have attracted interest as a photonic approach for the selective inactivation of virus contaminations in biological samples. Conventional pulsed laser approaches require, however, relatively long irradiation times to achieve a significant inactivation of virus. In this study, we investigate the enhancement of the photonic inactivation of Murine Leukemia Virus (MLV) via 805 nm femtosecond pulses through gold nanorods whose localized surface plasmon resonance overlaps with the excitation laser. We report a plasmonically enhanced virus inactivation, with greater than 3.7-log reduction measured by virus infectivity assays. Reliable virus inactivation was obtained for 10 s laser exposure with incident laser powers ≥0.3 W. Importantly, the fs-pulse induced inactivation was selective to the virus and did not induce any measurable damage to co-incubated antibodies. The loss in viral infection was associated with reduced viral fusion, linking the loss in infectivity with a perturbation of the viral envelope. Based on the observations that physical contact between nanorods and virus particles was not required for viral inactivation and that reactive oxygen species (ROS) did not participate in the detected viral inactivation, a model of virus inactivation based on plasmon enhanced shockwave generation is proposed.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Studies of inactivation mechanism of non-enveloped icosahedral virus by a visible ultrashort pulsed laser.Virol J. 2014 Feb 5;11:20. doi: 10.1186/1743-422X-11-20. Virol J. 2014. PMID: 24495489 Free PMC article.
-
Oxygen-dependent laser inactivation of murine norovirus using visible light lasers.Virol J. 2018 Jul 31;15(1):117. doi: 10.1186/s12985-018-1019-2. Virol J. 2018. PMID: 30064439 Free PMC article.
-
Accelerated inactivation of M13 bacteriophage using millijoule femtosecond lasers.J Biophotonics. 2020 Feb;13(2):e201900001. doi: 10.1002/jbio.201900001. Epub 2019 Nov 20. J Biophotonics. 2020. PMID: 31654474
-
Ultraviolet-C irradiation for inactivation of viruses in foetal bovine serum.Vaccine. 2018 Jul 5;36(29):4215-4221. doi: 10.1016/j.vaccine.2018.06.008. Epub 2018 Jun 8. Vaccine. 2018. PMID: 29891350
-
Comparative study on the inactivation of MS2 and M13 bacteriophages using energetic femtosecond lasers.J Biophotonics. 2020 Oct;13(10):e202000109. doi: 10.1002/jbio.202000109. Epub 2020 Aug 10. J Biophotonics. 2020. PMID: 32701195 Review.
Cited by
-
Surface interactions and viability of coronaviruses.J R Soc Interface. 2021 Jan;18(174):20200798. doi: 10.1098/rsif.2020.0798. Epub 2021 Jan 6. J R Soc Interface. 2021. PMID: 33402019 Free PMC article. Review.
-
A systemic review on liquid crystals, nanoformulations and its application for detection and treatment of SARS - CoV- 2 (COVID - 19).J Mol Liq. 2022 Sep 15;362:119795. doi: 10.1016/j.molliq.2022.119795. Epub 2022 Jul 8. J Mol Liq. 2022. PMID: 35832289 Free PMC article. Review.
-
Nanotechnology: A Potential Weapon to Fight against COVID-19.Part Part Syst Charact. 2022 Jan;39(1):2100159. doi: 10.1002/ppsc.202100159. Epub 2021 Nov 21. Part Part Syst Charact. 2022. PMID: 35440846 Free PMC article. Review.
-
Plasmonic nano-antimicrobials: properties, mechanisms and applications in microbe inactivation and sensing.Nanoscale. 2021 Feb 14;13(6):3374-3411. doi: 10.1039/d0nr08353d. Epub 2021 Feb 4. Nanoscale. 2021. PMID: 33538743 Free PMC article. Review.
-
Nanotechnology against COVID-19: Immunization, diagnostic and therapeutic studies.J Control Release. 2021 Aug 10;336:354-374. doi: 10.1016/j.jconrel.2021.06.036. Epub 2021 Jun 25. J Control Release. 2021. PMID: 34175366 Free PMC article. Review.
References
-
- Zharov VP, Letfullin RR, Galitovskaya EN. Microbubbles-overlapping mode for laser killing of cancer cells with absorbing nanoparticle cluster. J. Phys. D: Appl. Phys. 2005;38:2571. doi: 10.1088/0022-3727/38/15/007. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

