Efficacy and safety of sofosbuvir and daclatasvir in treatment of kidney transplantation recipients with hepatitis C virus infection
- PMID: 28932089
- PMCID: PMC5583582
- DOI: 10.3748/wjg.v23.i32.5969
Efficacy and safety of sofosbuvir and daclatasvir in treatment of kidney transplantation recipients with hepatitis C virus infection
Abstract
Aim: To assess the efficacy and safety of sofosbuvir and daclatasvir regimens for kidney transplantation (KT) patients with hepatitis C virus (HCV) infection.
Methods: This study enrolled a prospective cohort of consecutive Chinese KT patients with HCV infection. They were given sofosbuvir combined with daclatasvir, with or without ribavirin. They were monitored regularly during and after the treatment.
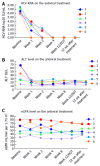
Results: Six patients were recruited in our prospective study cohort. All patients were male and naive to direct-acting antiviral treatment. The treatment duration was 12 wk. Most patients (4/6) were infected with HCV genotype 1b. HCV RNA was undetectable at week 4 after treatment and at the end of treatment in all patients. Sustained virological response rate at 12 wk was 100% (6/6). Two patients had to accept a half dose of sofosbuvir due to serum creatinine elevation during treatment. Kidney function in the remaining patients was stable. No serious adverse events (AEs) were observed. No patient discontinued antiviral therapy due to side effects.
Conclusion: Sofosbuvir and daclatasvir for treatment of KT recipients with HCV infection are highly efficient and safe. Patients tolerated the medications well, and no serious AEs were observed. Larger prospective cohort studies are needed to validate these results.
Keywords: Daclatasvir; Direct-acting antivirals; Hepatitis C virus; Kidney transplantation; Sofosbuvir.
Conflict of interest statement
Conflict-of-interest statement: All authors have no conflicts of interest.
Figures
References
-
- Fissell RB, Bragg-Gresham JL, Woods JD, Jadoul M, Gillespie B, Hedderwick SA, Rayner HC, Greenwood RN, Akiba T, Young EW. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2004;65:2335–2342. - PubMed
-
- Morales JM, Fabrizi F. Hepatitis C and its impact on renal transplantation. Nat Rev Nephrol. 2015;11:172–182. - PubMed
-
- EBPG (European Expert Group on Renal Transplantation); European Renal Association (ERA-EDTA); European Society for Organ Transplantation (ESOT) European Best Practice Guidelines for Renal Transplantation (part 1) Nephrol Dial Transplant. 2000;15 Suppl 7:1–85. - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008;(109):S1–S99. - PubMed
-
- Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, Lawitz E, Lok AS, Hinestrosa F, Thuluvath PJ, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211–221. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

