New botanical drug, HL tablet, reduces hepatic fat as measured by magnetic resonance spectroscopy in patients with nonalcoholic fatty liver disease: A placebo-controlled, randomized, phase II trial
- PMID: 28932090
- PMCID: PMC5583583
- DOI: 10.3748/wjg.v23.i32.5977
New botanical drug, HL tablet, reduces hepatic fat as measured by magnetic resonance spectroscopy in patients with nonalcoholic fatty liver disease: A placebo-controlled, randomized, phase II trial
Abstract
Aim: To evaluate the efficacy and safety of HL tablet extracted from magnolia officinalis for treating patients with nonalcoholic fatty liver disease (NAFLD).
Methods: Seventy-four patients with NAFLD diagnosed by ultrasonography were randomly assigned to 3 groups given high dose (400 mg) HL tablet, low dose (133.4 mg) HL tablet and placebo, respectively, daily for 12 wk. The primary endpoint was post-treatment change of hepatic fat content (HFC) measured by magnetic resonance spectroscopy. Secondary endpoints included changes of serum aspartate aminotransferase, alanine aminotransferase (ALT), cholesterol, triglyceride, free fatty acid, homeostasis model assessment-estimated insulin resistance, and body mass index (BMI).
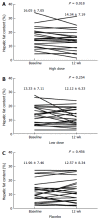
Results: The mean HFC of the high dose HL group, but not of the low dose group, declined significantly after 12 wk of treatment (high dose vs placebo, P = 0.033; low dose vs placebo, P = 0.386). The mean changes of HFC from baseline at week 12 were -1.7% ± 3.1% in the high dose group (P = 0.018), -1.21% ± 4.97% in the low dose group (P = 0.254) and 0.61% ± 3.87% in the placebo group (relative changes compared to baseline, high dose were: -12.1% ± 23.5%, low dose: -3.2% ± 32.0%, and placebo: 7.6% ± 44.0%). Serum ALT levels also tended to decrease in the groups receiving HL tablet while other factors were unaffected. There were no moderate or severe treatment-related safety issues during the study.
Conclusion: HL tablet is effective in reducing HFC without any negative lipid profiles, BMI changes and adverse effects.
Keywords: Botanical drug; Magnetic resonance spectroscopy; Magnolia officinali; Nonalcoholic fatty liver disease; Randomized controlled trial.
Conflict of interest statement
Conflict-of-interest statement: All authors have no conflicts of interest to disclose.
Figures
References
-
- Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. - PubMed
-
- Ahmed A, Wong RJ, Harrison SA. Nonalcoholic Fatty Liver Disease Review: Diagnosis, Treatment, and Outcomes. Clin Gastroenterol Hepatol. 2015;13:2062–2070. - PubMed
-
- Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. - PubMed
-
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. - PubMed
-
- Wong VW, Chan RS, Wong GL, Cheung BH, Chu WC, Yeung DK, Chim AM, Lai JW, Li LS, Sea MM, et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59:536–542. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical