Spermidine-mediated poly(lactic- co-glycolic acid) nanoparticles containing fluorofenidone for the treatment of idiopathic pulmonary fibrosis
- PMID: 28932114
- PMCID: PMC5598552
- DOI: 10.2147/IJN.S140569
Spermidine-mediated poly(lactic- co-glycolic acid) nanoparticles containing fluorofenidone for the treatment of idiopathic pulmonary fibrosis
Abstract
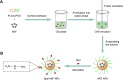
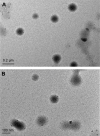
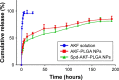
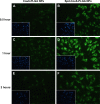
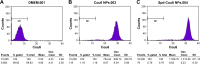
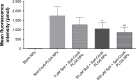
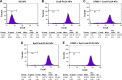
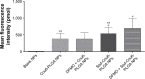
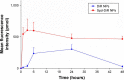
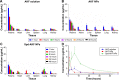
Idiopathic pulmonary fibrosis is a progressive, fatal lung disease with poor survival. The advances made in deciphering this disease have led to the approval of different antifibrotic molecules, such as pirfenidone and nintedanib. An increasing number of studies with particles (liposomes, nanoparticles [NPs], microspheres, nanopolymersomes, and nanoliposomes) modified with different functional groups have demonstrated improvement in lung-targeted drug delivery. In the present study, we prepared, characterized, and evaluated spermidine (Spd)-modified poly(lactic-co-glycolic acid) (PLGA) NPs as carriers for fluorofenidone (AKF) to improve the antifibrotic efficacy of this drug in the lung. Spd-AKF-PLGA NPs were prepared and functionalized by modified solvent evaporation with Spd and polyethylene glycol (PEG)-PLGA groups. The size of Spd-AKF-PLGA NPs was 172.5±4.3 nm. AKF release from NPs was shown to fit the Higuchi model. A549 cellular uptake of an Spd-coumarin (Cou)-6-PLGA NP group was found to be almost twice as high as that of the Cou-6-PLGA NP group. Free Spd and difluoromethylornithine (DFMO) were preincubated in A549 cells to prove uptake of Spd-Cou-6-PLGA NPs via a polyamine-transport system. As a result, the uptake of Spd-Cou-6-PLGA NPs significantly decreased with increased Spd concentrations in incubation. At higher Spd concentrations of 50 and 500 µM, uptake of Spd-Cou-6-PLGA NPs reduced 0.34- and 0.49-fold from that without Spd pretreatment. After pretreatment with DFMO for 36 hours, cellular uptake of Spd-Cou-6-PLGA NPs reached 1.26-fold compared to the untreated DFMO group. In a biodistribution study, the drug-targeting index of Spd-AKF-PLGA NPs in the lung was 3.62- and 4.66-fold that of AKF-PLGA NPs and AKF solution, respectively. This suggested that Spd-AKF-PLGA NPs accumulated effectively in the lung. Lung-histopathology changes and collagen deposition were observed by H&E staining and Masson staining in an efficacy study. In the Spd-AKF-PLGA NP group, damage was further improved compared to the AKF-PLGA NP group and AKF-solution group. The results indicated that Spd-AKF-PLGA NPs are able to be effective nanocarriers for anti-pulmonary fibrosis therapy.
Keywords: fluorofenidone; idiopathic pulmonary fibrosis; nanoparticles; polyamine transport system; spermidine.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures






Similar articles
-
Poly(lactide)-vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of Docetaxel.Biomaterials. 2009 Jul;30(19):3297-306. doi: 10.1016/j.biomaterials.2009.02.045. Epub 2009 Mar 19. Biomaterials. 2009. PMID: 19299012
-
Multifunctional nanoplatform based on star-shaped copolymer for liver cancer targeting therapy.Drug Deliv. 2019 Dec;26(1):595-603. doi: 10.1080/10717544.2019.1625467. Drug Deliv. 2019. PMID: 31195837 Free PMC article.
-
Co-delivery nanoparticles with characteristics of intracellular precision release drugs for overcoming multidrug resistance.Int J Nanomedicine. 2017 Mar 16;12:2081-2108. doi: 10.2147/IJN.S128790. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28356731 Free PMC article.
-
Biomedical applications of PLGA nanoparticles in nanomedicine: advances in drug delivery systems and cancer therapy.Expert Opin Drug Deliv. 2023 Jul-Dec;20(7):937-954. doi: 10.1080/17425247.2023.2223941. Epub 2023 Jun 23. Expert Opin Drug Deliv. 2023. PMID: 37294853 Review.
-
The effect of nanoparticle properties, detection method, delivery route and animal model on poly(lactic-co-glycolic) acid nanoparticles biodistribution in mice and rats.Drug Metab Rev. 2014 May;46(2):128-41. doi: 10.3109/03602532.2013.864664. Epub 2013 Dec 5. Drug Metab Rev. 2014. PMID: 24303927 Review.
Cited by
-
Afatinib-loaded inhalable PLGA nanoparticles for localized therapy of non-small cell lung cancer (NSCLC)-development and in-vitro efficacy.Drug Deliv Transl Res. 2021 Jun;11(3):927-943. doi: 10.1007/s13346-020-00802-8. Drug Deliv Transl Res. 2021. PMID: 32557351 Free PMC article.
-
A cross-tissue transcriptome-wide association study reveals GRK4 as a novel susceptibility gene for COPD.Sci Rep. 2024 Nov 18;14(1):28438. doi: 10.1038/s41598-024-80122-w. Sci Rep. 2024. PMID: 39558015 Free PMC article.
-
Exosomal miRNAs as biomarkers and therapeutic targets in silicosis-related lung fibrosis.Mol Biol Rep. 2025 Jun 12;52(1):585. doi: 10.1007/s11033-025-10687-w. Mol Biol Rep. 2025. PMID: 40504442 Free PMC article. Review.
-
Emerging drug delivery strategies for idiopathic pulmonary fibrosis treatment.Eur J Pharm Biopharm. 2021 Jul;164:1-12. doi: 10.1016/j.ejpb.2021.03.017. Epub 2021 Apr 18. Eur J Pharm Biopharm. 2021. PMID: 33882301 Free PMC article. Review.
-
Delivery strategies to improve the pharmacological efficacy of NRF2 modulators: a review.RSC Med Chem. 2025 Aug 22. doi: 10.1039/d5md00571j. Online ahead of print. RSC Med Chem. 2025. PMID: 40919319 Free PMC article. Review.
References
-
- Sgalla G, Cocconcelli E, Tonelli R, Richeldi L. Novel drug targets for idiopathic pulmonary fibrosis. Expert Rev Respir Med. 2016 Feb 26; Epub. - PubMed
-
- Xaubet A, Molina-Molina M, Acosta O, et al. Guidelines for the medical treatment of idiopathic pulmonary fibrosis. Arch Bronconeumol. 2017;53(5):263–269. - PubMed
-
- Spagnolo P, Wells AU, Collard HR. Pharmacological treatment of idiopathic pulmonary fibrosis: an update. Drug Discov Today. 2015;20(5):514–524. - PubMed
-
- Homer RJ, Elias JA, Lee CG, Herzog E. Modern concepts on the role of inflammation in pulmonary fibrosis. Arch Pathol Lab Med. 2011;135(6):780–788. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

