Is There Evidence for Myelin Modeling by Astrocytes in the Normal Adult Brain?
- PMID: 28932188
- PMCID: PMC5592641
- DOI: 10.3389/fnana.2017.00075
Is There Evidence for Myelin Modeling by Astrocytes in the Normal Adult Brain?
Abstract
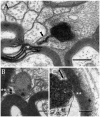
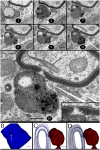
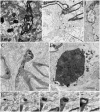
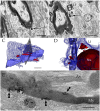
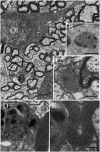
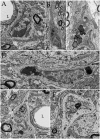
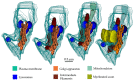
A set of astrocytic process associated with altered myelinated axons is described in the forebrain of normal adult rodents with confocal, electron microscopy, and 3D reconstructions. Each process consists of a protuberance that contains secretory organelles including numerous lysosomes which polarize and open next to disrupted myelinated axons. Because of the distinctive asymmetric organelle distribution and ubiquity throughout the forebrain neuropil, this enlargement is named paraxial process (PAP). The myelin envelope contiguous to the PAP displays focal disruption or disintegration. In routine electron microscopy clusters of large, confluent, lysosomes proved to be an effective landmark for PAP identification. In 3D assemblies lysosomes organize a series of interconnected saccules that open up to the plasmalemma next to the disrupted myelin envelope(s). Activity for acid hydrolases was visualized in lysosomes, and extracellularly at the PAP-myelin interface and/or between the glial and neuronal outer aspects. Organelles in astrocytic processes involved in digesting pyknotic cells and debris resemble those encountered in PAPs supporting a likewise lytic function of the later. Conversely, processes entangling tripartite synapses and glomeruli were devoid of lysosomes. Both oligodendrocytic and microglial processes were not associated with altered myelin envelopes. The possible roles of the PAP in myelin remodeling in the context of the oligodendrocyte-astrocyte interactions and in the astrocyte's secretory pathways are discussed.
Keywords: astrocytic process; lysosome; myelin remodeling; neuropil; secretion.
Figures







Similar articles
-
Perinodal astrocytic processes at nodes of Ranvier in developing normal and glial cell deficient rat spinal cord.Brain Res. 1985 Jul 1;337(2):321-31. doi: 10.1016/0006-8993(85)90069-1. Brain Res. 1985. PMID: 4027576
-
Fine Astrocyte Processes Contain Very Small Mitochondria: Glial Oxidative Capability May Fuel Transmitter Metabolism.Neurochem Res. 2015 Dec;40(12):2402-13. doi: 10.1007/s11064-015-1563-8. Epub 2015 Apr 18. Neurochem Res. 2015. PMID: 25894677
-
Myelinated nerve fibres in the CNS.Prog Neurobiol. 1993 Mar;40(3):319-84. doi: 10.1016/0301-0082(93)90015-k. Prog Neurobiol. 1993. PMID: 8441812 Review.
-
Ultrastructural and immunohistochemical analysis of axonal regrowth and myelination in membranes which form over lesion sites in the rat visual system.J Neurocytol. 1988 Dec;17(6):797-808. doi: 10.1007/BF01216707. J Neurocytol. 1988. PMID: 3230398
-
Local energy on demand: Are 'spontaneous' astrocytic Ca2+-microdomains the regulatory unit for astrocyte-neuron metabolic cooperation?Brain Res Bull. 2018 Jan;136:54-64. doi: 10.1016/j.brainresbull.2017.04.011. Epub 2017 Apr 24. Brain Res Bull. 2018. PMID: 28450076 Review.
Cited by
-
MERCs. The Novel Assistant to Neurotransmission?Front Neurosci. 2020 Nov 9;14:589319. doi: 10.3389/fnins.2020.589319. eCollection 2020. Front Neurosci. 2020. PMID: 33240039 Free PMC article. Review.
-
The roles of microglia and astrocytes in myelin phagocytosis in the central nervous system.J Cereb Blood Flow Metab. 2023 Mar;43(3):325-340. doi: 10.1177/0271678X221137762. Epub 2022 Nov 2. J Cereb Blood Flow Metab. 2023. PMID: 36324281 Free PMC article. Review.
References
-
- Bauman N., Pham-Dinh D. (2001). Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 81, 871–972. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

