Is Increased Intracellular Calcium in Red Blood Cells a Common Component in the Molecular Mechanism Causing Anemia?
- PMID: 28932200
- PMCID: PMC5592231
- DOI: 10.3389/fphys.2017.00673
Is Increased Intracellular Calcium in Red Blood Cells a Common Component in the Molecular Mechanism Causing Anemia?
Abstract
For many hereditary disorders, although the underlying genetic mutation may be known, the molecular mechanism leading to hemolytic anemia is still unclear and needs further investigation. Previous studies revealed an increased intracellular Ca2+ in red blood cells (RBCs) from patients with sickle cell disease, thalassemia, or Gardos channelopathy. Therefore we analyzed RBCs' Ca2+ content from 35 patients with different types of anemia (16 patients with hereditary spherocytosis, 11 patients with hereditary xerocytosis, 5 patients with enzymopathies, and 3 patients with hemolytic anemia of unknown cause). Intracellular Ca2+ in RBCs was measured by fluorescence microscopy using the fluorescent Ca2+ indicator Fluo-4 and subsequent single cell analysis. We found that in RBCs from patients with hereditary spherocytosis and hereditary xerocytosis the intracellular Ca2+ levels were significantly increased compared to healthy control samples. For enzymopathies and hemolytic anemia of unknown cause the intracellular Ca2+ levels in RBCs were not significantly different. These results lead us to the hypothesis that increased Ca2+ levels in RBCs are a shared component in the mechanism causing an accelerated clearance of RBCs from the blood stream in channelopathies such as hereditary xerocytosis and in diseases involving defects of cytoskeletal components like hereditary spherocytosis. Future drug developments should benefit from targeting Ca2+ entry mediating molecular players leading to better therapies for patients.
Keywords: calcium homeostasis; channelopathies; erythrocyte; live cell imaging; rare anemia; spherocytosis; xerocytosis.
Figures
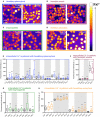
References
-
- Ataga K. I., Reid M., Ballas S. K., Yasin Z., Bigelow C., James L. S., et al. (2011). Improvements in haemolysis and indicators of erythrocyte survival do not correlate with acute vaso-occlusive crises in patients with sickle cell disease: a phase III randomized, placebo-controlled, double-blind study of the Gardos channel blocker senicapoc (ICA-17043). Br. J. Haematol. 153, 92–104. 10.1111/j.1365-2141.2010.08520.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

