Succinyl-CoA:Mesaconate CoA-Transferase and Mesaconyl-CoA Hydratase, Enzymes of the Methylaspartate Cycle in Haloarcula hispanica
- PMID: 28932214
- PMCID: PMC5592240
- DOI: 10.3389/fmicb.2017.01683
Succinyl-CoA:Mesaconate CoA-Transferase and Mesaconyl-CoA Hydratase, Enzymes of the Methylaspartate Cycle in Haloarcula hispanica
Abstract
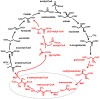
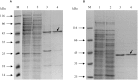
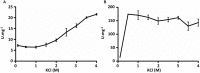
Growth on acetate or other acetyl-CoA-generating substrates as a sole source of carbon requires an anaplerotic pathway for the conversion of acetyl-CoA into cellular building blocks. Haloarchaea (class Halobacteria) possess two different anaplerotic pathways, the classical glyoxylate cycle and the novel methylaspartate cycle. The methylaspartate cycle was discovered in Haloarcula spp. and operates in ∼40% of sequenced haloarchaea. In this cycle, condensation of one molecule of acetyl-CoA with oxaloacetate gives rise to citrate, which is further converted to 2-oxoglutarate and then to glutamate. The following glutamate rearrangement and deamination lead to mesaconate (methylfumarate) that needs to be activated to mesaconyl-C1-CoA and hydrated to β-methylmalyl-CoA. The cleavage of β-methylmalyl-CoA results in the formation of propionyl-CoA and glyoxylate. The carboxylation of propionyl-CoA and the condensation of glyoxylate with another acetyl-CoA molecule give rise to two C4-dicarboxylic acids, thus regenerating the initial acetyl-CoA acceptor and forming malate, its final product. Here we studied two enzymes of the methylaspartate cycle from Haloarcula hispanica, succinyl-CoA:mesaconate CoA-transferase (mesaconate CoA-transferase, Hah_1336) and mesaconyl-CoA hydratase (Hah_1340). Their genes were heterologously expressed in Haloferax volcanii, and the corresponding enzymes were purified and characterized. Mesaconate CoA-transferase was specific for its physiological substrates, mesaconate and succinyl-CoA, and produced only mesaconyl-C1-CoA and no mesaconyl-C4-CoA. Mesaconyl-CoA hydratase had a 3.5-fold bias for the physiological substrate, mesaconyl-C1-CoA, compared to mesaconyl-C4-CoA, and virtually no activity with other tested enoyl-CoA/3-hydroxyacyl-CoA compounds. Our results further prove the functioning of the methylaspartate cycle in haloarchaea and suggest that mesaconate CoA-transferase and mesaconyl-CoA hydratase can be regarded as characteristic enzymes of this cycle.
Keywords: acetate assimilation; class III CoA-transferases; enoyl-CoA hydratases; haloarchaea; methylaspartate cycle.
Figures


Similar articles
-
Malate Synthase and β-Methylmalyl Coenzyme A Lyase Reactions in the Methylaspartate Cycle in Haloarcula hispanica.J Bacteriol. 2017 Jan 30;199(4):e00657-16. doi: 10.1128/JB.00657-16. Print 2017 Feb 15. J Bacteriol. 2017. PMID: 27920298 Free PMC article.
-
Identifying the missing steps of the autotrophic 3-hydroxypropionate CO2 fixation cycle in Chloroflexus aurantiacus.Proc Natl Acad Sci U S A. 2009 Dec 15;106(50):21317-22. doi: 10.1073/pnas.0908356106. Epub 2009 Dec 2. Proc Natl Acad Sci U S A. 2009. PMID: 19955419 Free PMC article.
-
The methylaspartate cycle in haloarchaea and its possible role in carbon metabolism.ISME J. 2016 Mar;10(3):546-57. doi: 10.1038/ismej.2015.132. Epub 2015 Aug 4. ISME J. 2016. PMID: 26241502 Free PMC article.
-
Unfamiliar metabolic links in the central carbon metabolism.J Biotechnol. 2014 Dec 20;192 Pt B:314-22. doi: 10.1016/j.jbiotec.2014.02.015. Epub 2014 Feb 24. J Biotechnol. 2014. PMID: 24576434 Review.
-
Biotechnological potential of the ethylmalonyl-CoA pathway.Appl Microbiol Biotechnol. 2011 Jan;89(1):17-25. doi: 10.1007/s00253-010-2873-z. Epub 2010 Sep 30. Appl Microbiol Biotechnol. 2011. PMID: 20882276 Review.
Cited by
-
Proteomic Analysis of Stationary Growth Stage Adaptation and Nutritional Deficiency Response of Brucella abortus.Front Microbiol. 2020 Dec 15;11:598797. doi: 10.3389/fmicb.2020.598797. eCollection 2020. Front Microbiol. 2020. PMID: 33384672 Free PMC article.
-
Redefining the coenzyme A transferase superfamily with a large set of manually annotated proteins.Protein Sci. 2022 Apr;31(4):864-881. doi: 10.1002/pro.4277. Epub 2022 Feb 7. Protein Sci. 2022. PMID: 35049101 Free PMC article.
-
Structural Basis for a Cork-Up Mechanism of the Intra-Molecular Mesaconyl-CoA Transferase.Biochemistry. 2023 Jan 3;62(1):75-84. doi: 10.1021/acs.biochem.2c00532. Epub 2022 Dec 19. Biochemistry. 2023. PMID: 36535006 Free PMC article.
-
TCA Cycle Replenishing Pathways in Photosynthetic Purple Non-Sulfur Bacteria Growing with Acetate.Life (Basel). 2021 Jul 19;11(7):711. doi: 10.3390/life11070711. Life (Basel). 2021. PMID: 34357087 Free PMC article. Review.
References
-
- Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A.et al. (1987). Current Protocols in Molecular Biology. New York: John Wiley.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

