Pertussis Maternal Immunization: Narrowing the Knowledge Gaps on the Duration of Transferred Protective Immunity and on Vaccination Frequency
- PMID: 28932228
- PMCID: PMC5592197
- DOI: 10.3389/fimmu.2017.01099
Pertussis Maternal Immunization: Narrowing the Knowledge Gaps on the Duration of Transferred Protective Immunity and on Vaccination Frequency
Abstract
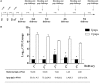
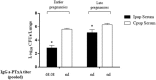
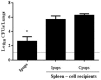
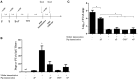
Maternal safety through pertussis vaccination and subsequent maternal-fetal-antibody transfer are well documented, but information on infant protection from pertussis by such antibodies and by subsequent vaccinations is scarce. Since mice are used extensively for maternal-vaccination studies, we adopted that model to narrow those gaps in our understanding of maternal pertussis immunization. Accordingly, we vaccinated female mice with commercial acellular pertussis (aP) vaccine and measured offspring protection against Bordetella pertussis challenge and specific-antibody levels with or without revaccination. Maternal immunization protected the offspring against pertussis, with that immune protection transferred to the offspring lasting for several weeks, as evidenced by a reduction (4-5 logs, p < 0.001) in the colony-forming-units recovered from the lungs of 16-week-old offspring. Moreover, maternal-vaccination-acquired immunity from the first pregnancy still conferred protection to offspring up to the fourth pregnancy. Under the conditions of our experimental protocol, protection to offspring from the aP-induced immunity is transferred both transplacentally and through breastfeeding. Adoptive-transfer experiments demonstrated that transferred antibodies were more responsible for the protection detected in offspring than transferred whole spleen cells. In contrast to reported findings, the protection transferred was not lost after the vaccination of infant mice with the same or other vaccine preparations, and conversely, the immunity transferred from mothers did not interfere with the protection conferred by infant vaccination with the same or different vaccines. These results indicated that aP-vaccine immunization of pregnant female mice conferred protective immunity that is transferred both transplacentally and via offspring breastfeeding without compromising the protection boostered by subsequent infant vaccination. These results-though admittedly not necessarily immediately extrapolatable to humans-nevertheless enabled us to test hypotheses under controlled conditions through detailed sampling and data collection. These findings will hopefully refine hypotheses that can then be validated in subsequent human studies.
Keywords: Bordetella pertussis; acellular vaccine; pertussis; pregnancy immunization; protection.
Figures


Similar articles
-
Maternal vaccination: shaping the neonatal response to pertussis.Front Immunol. 2023 Jul 12;14:1210580. doi: 10.3389/fimmu.2023.1210580. eCollection 2023. Front Immunol. 2023. PMID: 37520565 Free PMC article. Review.
-
Use of a Neonatal-Mouse Model to Characterize Vaccines and Strategies for Overcoming the High Susceptibility and Severity of Pertussis in Early Life.Front Microbiol. 2020 Apr 17;11:723. doi: 10.3389/fmicb.2020.00723. eCollection 2020. Front Microbiol. 2020. PMID: 32362890 Free PMC article.
-
Impact of maternal whole-cell or acellular pertussis primary immunization on neonatal immune response.Front Immunol. 2023 Jun 26;14:1192119. doi: 10.3389/fimmu.2023.1192119. eCollection 2023. Front Immunol. 2023. PMID: 37435078 Free PMC article.
-
Reciprocal interference of maternal and infant immunization in protection against pertussis.Vaccine. 2016 Feb 17;34(8):1062-9. doi: 10.1016/j.vaccine.2016.01.011. Epub 2016 Jan 15. Vaccine. 2016. PMID: 26776471
-
Acellular pertussis vaccine use in risk groups (adolescents, pregnant women, newborns and health care workers): a review of evidences and recommendations.Vaccine. 2012 Jul 27;30(35):5179-90. doi: 10.1016/j.vaccine.2012.06.005. Epub 2012 Jun 15. Vaccine. 2012. PMID: 22709953 Review.
Cited by
-
Maternal vaccination: shaping the neonatal response to pertussis.Front Immunol. 2023 Jul 12;14:1210580. doi: 10.3389/fimmu.2023.1210580. eCollection 2023. Front Immunol. 2023. PMID: 37520565 Free PMC article. Review.
-
Use of a Neonatal-Mouse Model to Characterize Vaccines and Strategies for Overcoming the High Susceptibility and Severity of Pertussis in Early Life.Front Microbiol. 2020 Apr 17;11:723. doi: 10.3389/fmicb.2020.00723. eCollection 2020. Front Microbiol. 2020. PMID: 32362890 Free PMC article.
-
Immunomodulation as a Novel Strategy for Prevention and Treatment of Bordetella spp. Infections.Front Immunol. 2019 Dec 13;10:2869. doi: 10.3389/fimmu.2019.02869. eCollection 2019. Front Immunol. 2019. PMID: 31921136 Free PMC article. Review.
-
Impact of maternal whole-cell or acellular pertussis primary immunization on neonatal immune response.Front Immunol. 2023 Jun 26;14:1192119. doi: 10.3389/fimmu.2023.1192119. eCollection 2023. Front Immunol. 2023. PMID: 37435078 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

