Peak nasal inspiratory flow as outcome for provocation studies in allergen exposure chambers: a GA2LEN study
- PMID: 28932387
- PMCID: PMC5604509
- DOI: 10.1186/s13601-017-0169-4
Peak nasal inspiratory flow as outcome for provocation studies in allergen exposure chambers: a GA2LEN study
Abstract
Background: The GA2LEN chamber has been developed as a novel mobile allergen exposure chamber (AEC) allowing standardized multicenter trials in allergy. Hitherto, subjective nasal symptom scores have been the most often used outcome parameter, but in standardized modern trials objective parameters are preferred. Despite its practicability, the objective parameter peak nasal inspiratory flow (PNIF) has been rarely used for allergy trials in the setting of allergen exposure chambers. This study aims to evaluate PNIF as an outcome parameter for provocation studies in AECs.
Methods: In a randomized controlled blinded setting subjects suffering from allergic rhinitis were exposed to grass pollen, birch pollen, house dust mite and/or placebo in the GA2LEN chamber. Different allergen concentrations were used to evaluate symptom severities. Patients had to perform PNIF before and every 30 min during a challenge using a portable PNIF meter.
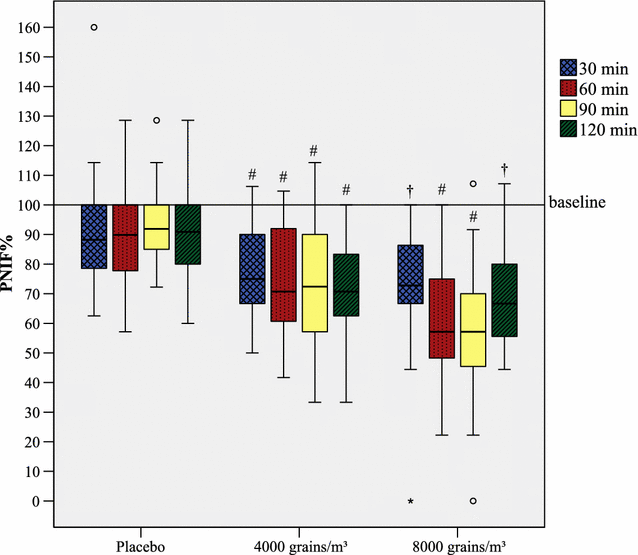
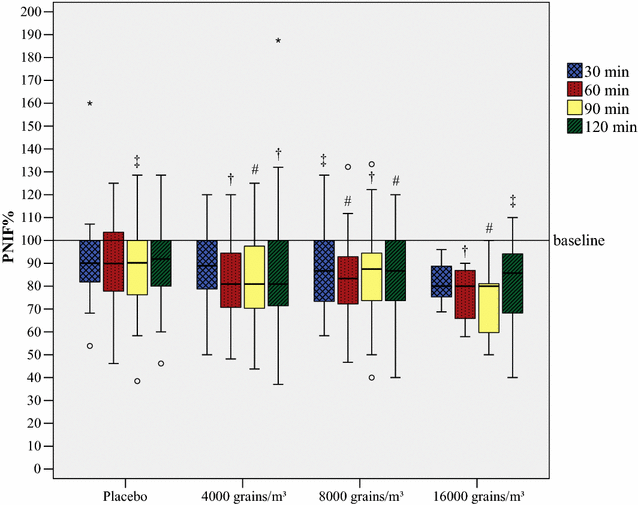
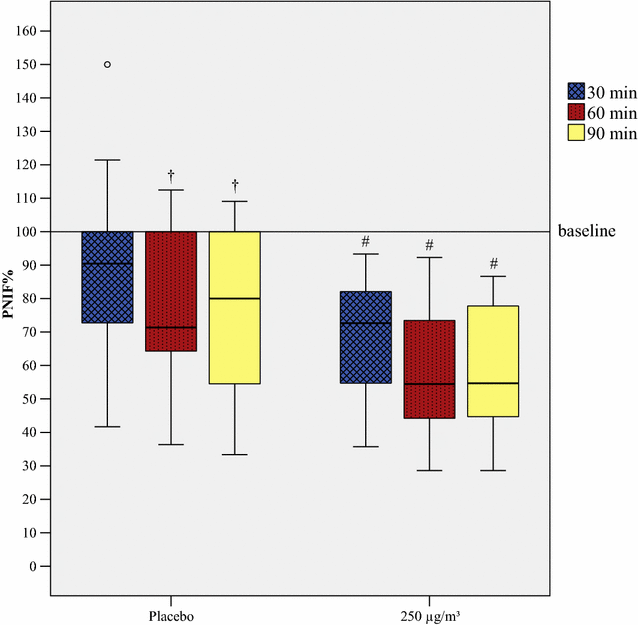
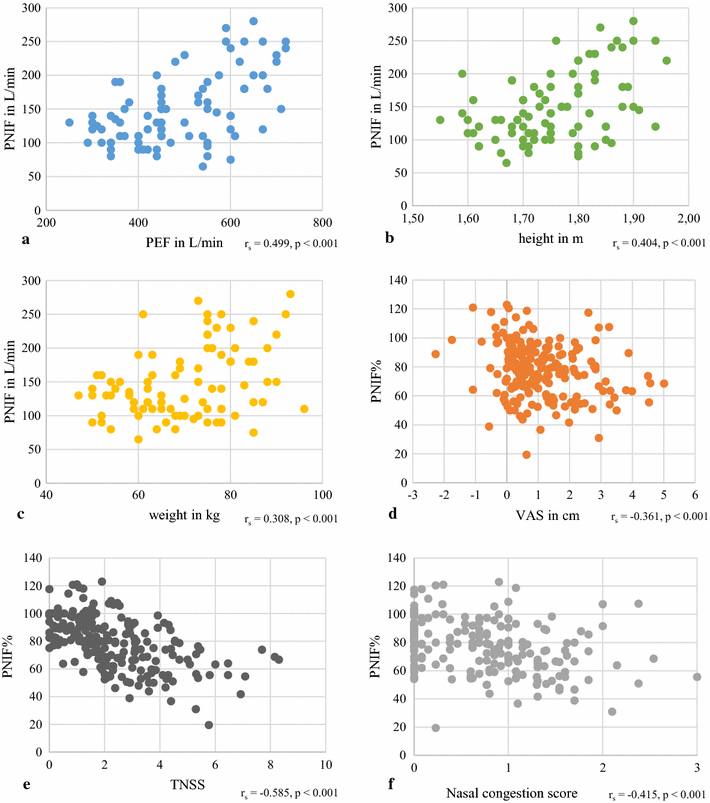
Results: 86 subjects participated in 203 challenges, altogether. House dust mite provocations caused the greatest reduction in PNIF values, followed by grass pollen and birch pollen. Provocations with every allergen or pollen concentration led to a significant decrease (p < 0.05) in PNIF compared to baseline. Furthermore, positive correlations were obtained between PNIF and peak expiratory flow, height and weight, and inverse correlations between PNIF and total nasal symptom score, nasal congestion score and visual analog scale of overall subjective symptoms.
Conclusion: PNIF is a helpful and feasible tool for conducting provocation trials with allergens, especially grass pollen and house dust mite, in an AEC.
Keywords: Allergen exposure chamber (AEC); Allergy trial; GA2LEN chamber; Peak nasal inspiratory flow (PNIF); Provocation study.
Figures
References
-
- Pefura-Yone EW, Kengne AP, Balkissou AD, Boulleys-Nana JR, Efe-de-Melingui NR, Ndjeutcheu-Moualeu PI, Mbele-Onana CL, Kenmegne-Noumsi EC, Kolontchang-Yomi BL, Theubo-Kamgang BJ, et al. Prevalence of asthma and allergic rhinitis among adults in Yaounde, Cameroon. PLoS ONE. 2015;10:e0123099. doi: 10.1371/journal.pone.0123099. - DOI - PMC - PubMed
-
- Salo PM, Arbes SJ, Jaramillo R, Calatroni A, Weir CH, Sever ML, Hoppin JA, Rose KM, Liu AH, Gergen PJ, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005–2006. J Allergy Clin Immunol. 2014;134:350–359. doi: 10.1016/j.jaci.2013.12.1071. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

