Crystal structures of three 1-[4-(4-bromo-but-oxy)-phen-yl] chalcone derivatives: (E)-1-[4-(4-bromo-but-oxy)-phen-yl]-3-phenyl-prop-2-en-1-one, (E)-1-[4-(4-bromo-but-oxy)-phen-yl]-3-(4-meth-oxy-phen-yl)prop-2-en-1-one and (E)-1-[4-(4-bromo-but-oxy)-phen-yl]-3-(3,4-di-meth-oxy-phen-yl)prop-2-en-1-one
- PMID: 28932443
- PMCID: PMC5598855
- DOI: 10.1107/S2056989017010052
Crystal structures of three 1-[4-(4-bromo-but-oxy)-phen-yl] chalcone derivatives: (E)-1-[4-(4-bromo-but-oxy)-phen-yl]-3-phenyl-prop-2-en-1-one, (E)-1-[4-(4-bromo-but-oxy)-phen-yl]-3-(4-meth-oxy-phen-yl)prop-2-en-1-one and (E)-1-[4-(4-bromo-but-oxy)-phen-yl]-3-(3,4-di-meth-oxy-phen-yl)prop-2-en-1-one
Abstract
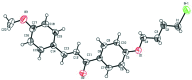
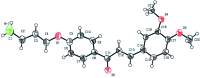
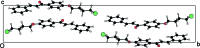
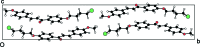
The crystal structures of three chalcones with a bromo-substituted but-oxy side chain, viz. (E)-1-[4-(4-bromo-but-oxy)-phen-yl]-3-phenyl-prop-2-en-1-one, C19H19BrO2, (I), (E)-1-[4-(4-bromo-but-oxy)-phen-yl]-3-(4-meth-oxy-phen-yl)prop-2-en-1-one, C20H21BrO3, (II), and (E)-1-[4-(4-bromo-but-oxy)-phen-yl]-3-(3,4-di-meth-oxy-phen-yl)prop-2-en-1-one, C21H23BrO4, (III), are reported. In all mol-ecules, the conformation of the keto group with respect to the olefinic bond is s-cis. Mol-ecules of (I) and (II) are nearly planar, while mol-ecule (III) is not planar. In the crystal of compounds (I) and (II), mol-ecules are linked into chains parallel to the c axis by C-H⋯π inter-actions. In the crystal of compound (III), mol-ecules are linked by a pairs of C-H⋯O hydrogen bonds, forming inversion dimers. Weak C-Br⋯π inter-actions are also observed in (III).
Keywords: Claisen–Schmidt condensation reaction; C—Br⋯π contact; chalcones; crystal structures; hydrogen bonding; molecular conformation.
Figures

References
-
- Aponte, J. C., Verástegui, M., Málaga, E., Zimic, M., Quiliano, M., Vaisberg, A. J., Gilman, R. H. & Hammond, G. B. (2008). J. Med. Chem. 51, 6230–6234. - PubMed
-
- Bappaliage, N. N., Narayana, Y., Poojary, B. & Poojary, K. N. (2010). IJPAP, 6, 151–156.
-
- Barot, V. M., Sahaj, A. G., Mahato, A. & Mehta, N. B. (2013). IJSRP, 3, 737–740.
-
- Bello, M. L., Chiaradia, L. M., Dias, L. R. S., Pacheco, L. K., Stumpf, T. R., Mascarello, A., Steindel, M., Yunes, R. A., Castro, H. C., Nunes, R. J. & Rodrigues, C. R. (2011). Bioorg. Med. Chem. 19, 5046–5052. - PubMed
-
- Bruker (2004). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
