Crystal structure of bis-[ N-(2-hy-droxy-eth-yl)- N-methyl-dithio-carbamato-κ2S, S'](pyridine)-zinc(II) pyridine monosolvate and its N-ethyl analogue
- PMID: 28932446
- PMCID: PMC5598858
- DOI: 10.1107/S2056989017010568
Crystal structure of bis-[ N-(2-hy-droxy-eth-yl)- N-methyl-dithio-carbamato-κ2S, S'](pyridine)-zinc(II) pyridine monosolvate and its N-ethyl analogue
Abstract
The common structural feature of the title compounds, [Zn(C4H8NOS2)2(C5H5N)]·C5H5N (I) and [Zn(C5H10NOS2)2(C5H5N)]·C5H5N (II), which differ by having di-thio-carbamate N-bound methyl (I) and ethyl (II) groups, is the coordination of each ZnII atom by two non-symmetrically chelating di-thio-carbamate ligands and by a pyridine ligand; in each case, the non-coordinating pyridine mol-ecule is connected to the Zn-containing mol-ecule via a (hy-droxy)O-H⋯N(pyridine) hydrogen bond. The resulting NS4 coordination geometry is closer to a square-pyramid than a trigonal bipyramid in the case of (I), but almost inter-mediate between the two extremes in (II). The mol-ecular packing features (hy-droxy)O-H⋯O(hy-droxy) hydrogen bonds, leading to supra-molecular chains with a zigzag arrangement along [10-1] (I) or a helical arrangement along [010] (II). In (I), π-π [inter-centroid distances = 3.4738 (10) and 3.4848 (10) Å] between coordinating and non-coordinating pyridine mol-ecules lead to stacks comprising alternating rings along the a axis. In (II), weaker π-π contacts occur between centrosymmetrically related pairs of coordinating pyridine mol-ecules [inter-centroid separation = 3.9815 (14) Å]. Further inter-actions, including C-H⋯π(chelate) inter-actions in (I), lead to a three-dimensional architecture in each case.
Keywords: crystal structure; dithiocarbamate; hydrogen bonding; pyridine adduct; zinc.
Figures

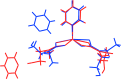
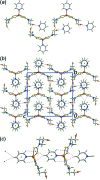
 ] and sustained by O—H⋯O hydrogen bonding, with the solvent pyridine molecules attached via O—H⋯N hydrogen bonding, (b) a view of the unit-cell contents in projection down the a axis and (c) supramolecular chain along the b axis sustained by (pyridine)C—H⋯π(chelate ring) interactions. The O—H⋯O, O—H⋯N, C—H⋯O, π–π and C—H⋯π(chelate ring) interactions are shown as orange, blue, brown, purple and pink dashed lines, respectively.
] and sustained by O—H⋯O hydrogen bonding, with the solvent pyridine molecules attached via O—H⋯N hydrogen bonding, (b) a view of the unit-cell contents in projection down the a axis and (c) supramolecular chain along the b axis sustained by (pyridine)C—H⋯π(chelate ring) interactions. The O—H⋯O, O—H⋯N, C—H⋯O, π–π and C—H⋯π(chelate ring) interactions are shown as orange, blue, brown, purple and pink dashed lines, respectively.
References
-
- Addison, A. W., Rao, T. N., Reedijk, J., van Rijn, J. & Verschoor, G. C. (1984). J. Chem. Soc. Dalton Trans. pp. 1349–1356.
-
- Benson, R. E., Ellis, C. A., Lewis, C. E. & Tiekink, E. R. T. (2007). CrystEngComm, 9, 930–941.
-
- Brandenburg, K. (2006). DIAMOND. Crystal Impact GbR, Bonn, Germany.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
