A novel in silico framework to improve MHC-I epitopes and break the tolerance to melanoma
- PMID: 28932628
- PMCID: PMC5599093
- DOI: 10.1080/2162402X.2017.1319028
A novel in silico framework to improve MHC-I epitopes and break the tolerance to melanoma
Erratum in
-
Corrigendum.Oncoimmunology. 2017 Oct 6;6(10):e1389146. doi: 10.1080/2162402X.2017.1389146. eCollection 2017. Oncoimmunology. 2017. PMID: 29123968 Free PMC article.
Abstract
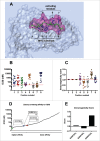
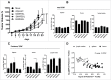
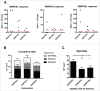
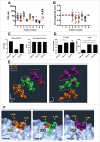
Tolerance toward tumor antigens, which are shared by normal tissues, have often limited the efficacy of cancer vaccines. However, wild type epitopes can be tweaked to activate cross-reactive T-cell clones, resulting in antitumor activity. The design of these analogs (i.e., heteroclitic peptides) can be difficult and time-consuming since no automated in silico tools are available. Hereby we describe the development of an in silico framework to improve the selection of heteroclitic peptides. The Epitope Discovery and Improvement System (EDIS) was first validated by studying the model antigen SIINFEKL. Based on artificial neural network (ANN) predictions, we selected two mutant analogs that are characterized by an increased MHC-I binding affinity (SIINFAKL) or increased TCR stimulation (SIIWFEKL). Therapeutic vaccination using optimized peptides resulted in enhanced antitumor activity and against B16.OVA melanomas in vivo. The translational potential of the EDIS platform was further demonstrated by studying the melanoma-associated antigen tyrosinase related protein 2 (TRP2). Following therapeutic immunization with the EDIS-derived epitope SVYDFFAWL, a significant reduction in the growth of established B16.F10 tumors was observed, suggesting a break in the tolerance toward the wild type epitope. Finally, we tested a multi vaccine approach, demonstrating that combination of wild type and mutant epitopes targeting both TRP2 and OVA antigens increases the antitumor response. In conclusion, by taking advantage of available prediction servers and molecular dynamics simulations, we generated an innovative platform for studying the initial sequences and selecting lead candidates with improved immunological features. Taken together, EDIS is the first automated algorithm-driven platform to speed up the design of heteroclitic peptides that can be publicly queried online.
Keywords: Cancer vaccine; heteroclitic peptides; immunotherapy; in silico; prediction servers; tumor antigens.
Figures


Similar articles
-
Dendritic cells break tolerance and induce protective immunity against a melanocyte differentiation antigen in an autologous melanoma model.Cancer Res. 2000 Dec 15;60(24):6995-7001. Cancer Res. 2000. PMID: 11156402
-
Multi-Epitope DC Vaccines with Melanoma Antigens for Immunotherapy of Melanoma.Vaccines (Basel). 2025 Mar 25;13(4):346. doi: 10.3390/vaccines13040346. Vaccines (Basel). 2025. PMID: 40333215 Free PMC article.
-
Therapy of established B16-F10 melanoma tumors by a single vaccination of CTL/T helper peptides in VacciMax.J Transl Med. 2007 Apr 23;5:20. doi: 10.1186/1479-5876-5-20. J Transl Med. 2007. PMID: 17451606 Free PMC article.
-
A modified tyrosinase-related protein 2 epitope generates high-affinity tumor-specific T cells but does not mediate therapeutic efficacy in an intradermal tumor model.J Immunol. 2006 Jul 1;177(1):155-61. doi: 10.4049/jimmunol.177.1.155. J Immunol. 2006. PMID: 16785510
-
New Immunoinformatics Tools for Swine: Designing Epitope-Driven Vaccines, Predicting Vaccine Efficacy, and Making Vaccines on Demand.Front Immunol. 2020 Oct 5;11:563362. doi: 10.3389/fimmu.2020.563362. eCollection 2020. Front Immunol. 2020. PMID: 33123135 Free PMC article. Review.
Cited by
-
Structural and Dynamic-Based Characterization of the Recognition Patterns of E7 and TRP-2 Epitopes by MHC Class I Receptors through Computational Approaches.Int J Mol Sci. 2024 Jan 23;25(3):1384. doi: 10.3390/ijms25031384. Int J Mol Sci. 2024. PMID: 38338663 Free PMC article.
-
Novel Insights Into Mesothelioma Therapy: Emerging Avenues and Future Prospects.Front Oncol. 2022 Jun 17;12:916839. doi: 10.3389/fonc.2022.916839. eCollection 2022. Front Oncol. 2022. PMID: 35785199 Free PMC article. Review.
-
Optimization of Early Steps in Oncolytic Adenovirus ONCOS-401 Production in T-175 and HYPERFlasks.Int J Mol Sci. 2019 Jan 31;20(3):621. doi: 10.3390/ijms20030621. Int J Mol Sci. 2019. PMID: 30709038 Free PMC article.
-
Prognostic Comparison between cTACE and H101-TACE in Unresectable Hepatocellular Carcinoma (HCC): A Propensity-Score Matching Analysis.Appl Bionics Biomech. 2022 Sep 2;2022:9084852. doi: 10.1155/2022/9084852. eCollection 2022. Appl Bionics Biomech. 2022. Retraction in: Appl Bionics Biomech. 2023 Dec 20;2023:9813798. doi: 10.1155/2023/9813798. PMID: 36091626 Free PMC article. Retracted.
-
Prospects of Replication-Deficient Adenovirus Based Vaccine Development against SARS-CoV-2.Vaccines (Basel). 2020 Jun 10;8(2):293. doi: 10.3390/vaccines8020293. Vaccines (Basel). 2020. PMID: 32531955 Free PMC article.
References
-
- Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R et al.. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 2012. August; 18(8):1254–61; PMID: 22842478; https://doi.org/10.1038/nm.2883 - DOI - PubMed
-
- Vacchelli E, Martins I, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: peptide vaccines in cancer therapy. Oncoimmunology 2012; 1(9):1557-76; PMID: 23264902; https://doi.org/10.4161/onci.22428 - DOI - PMC - PubMed
-
- Chianese-Bullock KA, Pressley J, Garbee C, Hibbitts S, Murphy C, Yamshchikov G, Petroni GR, Bissonette EA, Neese PY, Grosh WW et al.. MAGE-A1-, MAGE-A10-, and gp100-derived peptides are immunogenic when combined with granulocyte-macrophage colony-stimulating factor and montanide ISA-51 adjuvant and administered as part of a multipeptide vaccine for melanoma. J Immunol 2005; 174(5):3080-6; PMID: 15728523; https://doi.org/10.4049/jimmunol.174.5.3080 - DOI - PubMed
-
- Menez-Jamet J, Gallou C, Rougeot A, Kosmatopoulos K. Optimized tumor cryptic peptides: the basis for universal neo-antigen-like tumor vaccines. Ann Transl Med 2016; 4(14):266; PMID: 27563653; https://doi.org/10.21037/atm.2016.05.15 - DOI - PMC - PubMed
-
- Hebeisen M, Allard M, Gannon PO, Schmidt J, Speiser DE, Rufer N. Identifying individual T Cell receptors of optimal avidity for tumor antigens. Front Immunol 2015; 6:582; PMID: 26635796; https://doi.org/10.3389/fimmu.2015.00582 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
