The safety, efficacy, and treatment outcomes of a combination of low-dose decitabine treatment in patients with recurrent ovarian cancer
- PMID: 28932630
- PMCID: PMC5599090
- DOI: 10.1080/2162402X.2017.1323619
The safety, efficacy, and treatment outcomes of a combination of low-dose decitabine treatment in patients with recurrent ovarian cancer
Abstract
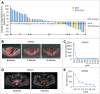
Purpose: DNA demethylating agents have shown clinical effectiveness in hematological and solid tumors. This trial tested the safety, efficacy, and treatment outcomes of decitabine-based chemotherapy or combined with immunotherapy in recurrent ovarian cancer patients. Patients and methods: Fifty-five patients with recurrent ovarian cancer were enrolled and 52 were assessable for clinical response and survival. Patients either received 5-d decitabine treatment, followed by reduced-dose of paclitaxel/carboplatin administration (DTC cohort), or the aforementioned regimen combined with cytokine-induced killer cells therapy (DTC+CIK cohort). The primary end point was clinical response rate and progression-free survival (PFS). Secondary evaluation included safety assessment and overall survival (OS). Results: Disease control rate (DCR) and objective response rate (ORR) were 73.91% and 23.91% in disease measurable patients by RECIST criteria, totally 76.92% and 30.77%, including disease non-measurable patients, which were higher in platinum-resistant/refractory patients. Clinical benefits could be associated with the number of DAC treatment cycles and the inclusion of CIK immunotherapy. In DTC+CIK cohort, DCR and ORR reached 100% and 58.30%, respectively. Notably, DTC+CIK treatment in platinum-resistant/refractory patients had an ORR of 87.50%. Consistently, PFS was longer in platinum-resistant/refractory patients comparing with that of platinum-sensitive patients. PFS and OS were 8 and 19 mo in platinum-resistant/refractory patients with DTC+CIK therapy. The most common toxicities were nausea, anorexia, fatigue, neutropenia, and anemia; many of which were grade 1-2. Conclusion: Low-dose DAC/paclitaxel/carboplatin regimen demonstrates disease benefit, especially in patients with platinum-resistant/refractory ovarian cancer, and might show remarkable clinical response when combined with adoptive immunotherapy in platinum-resistant/refractory ovarian cancer patients.
Keywords: CIK therapy; decitabine; epigenetic therapy; platinum sensitivity; recurrent ovarian cancer.
Figures


References
-
- Thigpen JT, Blessing JA, Ball H, Hummel SJ, Barrett RJ. Phase II trial of paclitaxel in patients with progressive ovarian carcinoma after platinum-based chemotherapy: A gynecologic oncology group study. J Clin Oncol 1994; 12:1748-53; PMID:7916038; https://doi.org/10.1200/JCO.1994.12.9.1748 - DOI - PubMed
-
- Pujade-Lauraine E. How to approach patients in relapse. Ann Oncol 2012; 23(Suppl 10):x128-31; PMID:22987947; https://doi.org/10.1093/annonc/mds358 - DOI - PubMed
-
- Markman M, Rothman R, Hakes T, Reichman B, Hoskins W, Rubin S, Jones W, Almadrones L, Lewis JL Jr. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol 1991; 9:389-93; PMID:1999708; https://doi.org/10.1200/JCO.1991.9.3.389 - DOI - PubMed
-
- Cannistra SA. Is there a “best” choice of second-line agent in the treatment of recurrent, potentially platinum-sensitive ovarian cancer? J Clin Oncol 2002; 20:1158-60; PMID:11870154; https://doi.org/10.1200/JCO.2002.20.5.1158 - DOI - PubMed
-
- Vaughan S, Coward JI, Bast RC Jr., Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D et al.. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat Rev Cancer 2011; 11:719-25; PMID:21941283; https://doi.org/10.1038/nrc3144 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
