Romidepsin alone or in combination with anti-CD20 chimeric antigen receptor expanded natural killer cells targeting Burkitt lymphoma in vitro and in immunodeficient mice
- PMID: 28932644
- PMCID: PMC5599075
- DOI: 10.1080/2162402X.2017.1341031
Romidepsin alone or in combination with anti-CD20 chimeric antigen receptor expanded natural killer cells targeting Burkitt lymphoma in vitro and in immunodeficient mice
Abstract
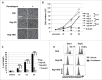
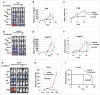
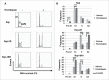
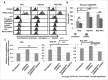
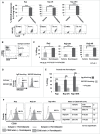
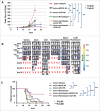
Facilitating the development of alternative targeted therapeutic strategies is urgently required to improve outcome or circumvent chemotherapy resistance in children, adolescents, and adults with recurrent/refractory de novo mature B-cell (CD20) non-Hodgkin lymphoma, including Burkitt lymphoma (BL). Romidepsin, a histone deacetylase inhibitor (HDACi), has been used to treat cutaneous T-cell lymphoma. We have demonstrated the significant anti-tumor effect of anti-CD20 chimeric antigen receptor (CAR) modified expanded peripheral blood natural killer (exPBNK) against rituximab-sensitive and -resistant BL. This study examined the anti-tumor activity of romidepsin alone and in combination with anti-CD20 CAR exPBNKs against rituximab-sensitive and -resistant BL in vitro and in vivo. We found that romidepsin significantly inhibited both rituximab-sensitive and -resistant BL cell proliferation in vitro (P < 0.001) and induced cell death in rituximab-sensitive Raji (P < 0.001) and cell cycle arrest in rituximab-resistant Raji-2R and Raji-4RH (P < 0.001). Consistent with in vitro observations, we also found romidepsin significantly inhibited the growth of rituximab-sensitive and -resistant BL in BL xenografted NSG mice. We also demonstrated that romidpesin significantly induced the expression of Natural Killer Group 2, Member D (NKG2D) ligands MICA/B in both rituximab-sensitive and -resistant BL cells (P < 0.001) resulting in enhancement of exPBNK in vitro cytotoxicity through NKG2D. Finally, we observed the combination of romidepsin and anti-CD20 CAR exPBNK significantly induced cell death in BL cells in vitro, reduced tumor burden and enhanced survival in humanized BL xenografted NSG mice (p < 0.05). Our data suggests that romidepsin is an active HDAC inhibitor that also potentiates expanded NK and anti-CD20 CAR exPBNK activity against rituximab-sensitive and -resistant BL.
Keywords: anti-CD20 chimeric antigen receptor; expanded Natural Killer Cells; rituximab sensitive and resistant Burkitt Lymphoma; romidepsin; targeted immunotherapy.
Figures

References
-
- Pinkerton R, Cairo MS. Childhood Non-Hodgkin Lymphoma In: Cairo MS, Perkins SL, eds. Hematological Malignancies in Children, Adolescents and Young Adults. Singapore: World Scientific, 2012:299-328; ISBN:978-981-4299-60-2; https://doi.org/ 10.1142/9789814299619_0016 - DOI
-
- Cairo MS, Gerrard M, Sposto R, Auperin A, Pinkerton CR, Michon J, Weston C, Perkins SL, Raphael M, McCarthy K, et al.. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood 2007; 109:2736-43; PMID:17138821; https://doi.org/ 10.1182/blood-2006-07-036665 - DOI - PMC - PubMed
-
- Gerrard M, Cairo MS, Weston C, Auperin A, Pinkerton R, Lambilliote A, Sposto R, McCarthy K, Lacombe MJ, Perkins SL, et al.. Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin's lymphoma: results of the FAB/LMB 96 international study. Br J Haematol 2008; 141:840-7; PMID:18371107; https://doi.org/ 10.1111/j.1365-2141.2008.07144.x - DOI - PubMed
-
- Cairo MS, Sposto R, Gerrard M, Auperin A, Goldman SC, Harrison L, Pinkerton R, Raphael M, McCarthy K, Perkins SL, et al.. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (>/ = 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin's lymphoma: results of the FAB LMB 96 study. J Clin Oncol 2012; 30:387-93; PMID:22215753; https://doi.org/ 10.1200/JCO.2010.33.3369 - DOI - PMC - PubMed
-
- Hoelzer D, Walewski J, Dohner H, Viardot A, Hiddemann W, Spiekermann K, Serve H, Dührsen U, Hüttmann A, Thiel E, et al.. Improved outcome of adult Burkitt lymphoma/leukemia with rituximab and chemotherapy: report of a large prospective multicenter trial. Blood 2014; 124:3870-9; PMID:25359988; https://doi.org/ 10.1182/blood-2014-03-563627 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
