Evaluation of a new immunoassay for chromogranin A measurement on the Kryptor system
- PMID: 28932793
- PMCID: PMC5597707
- DOI: 10.1016/j.plabm.2015.03.002
Evaluation of a new immunoassay for chromogranin A measurement on the Kryptor system
Abstract
Background: Chromogranin A (CgA) is a biomarker for neuroendocrine tumors (NETs). The aims of this study were to evaluate differences in measurement between the ThermoFisher Brahms CgA Kryptor assay and the CisBio assay and to investigate the influence of patient covariates. Temperature stability of CgA using both assays was determined.
Design and methods: 406 patients were analyzed for serum CgA using both assays. We performed a comparison study to determine whether several patient covariates (gender, use of protein pump inhibitors, impaired kidney function, referral department and tumor location) influenced the results. For the stability study, pooled serum samples were aliquoted and stored at different storage temperatures (room temperature, 4 °C and -20 °C) until assayed. In addition, 15 individual samples were evaluated after storage at 4 °C using the Kryptor assay.
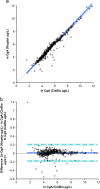
Results: Differences in measured concentrations between the assays were statistically significant. Passing & Bablok fit showed ln Y(Kryptor)=1.05 ln X(CisBio) - 0.20 with a bias of 1.0% after logarithmic transformation. Patient covariates were not associated. Patients׳ sera showed variable stability for CgA in the Kryptor assay at room temperature and 4 °C, whereas the recovery in the CisBio assay was stable at both temperatures.
Conclusion: Differences in measured CgA concentration between the assays could not be explained by the investigated patient covariates. Serum should be stored at -20 °C prior to determination using the Kryptor assay.
Keywords: CgA, chromogranin A; Chromogranin A methods; ELISA, enzyme-linked immuno sorbent assay; ENETS, European Neuroendocrine Tumor Society; GEP-NET, gastroentropancreatic NET; H2RA, H2-receptor antagonist; LD, lactate dehydrogenase; MDRD, modification of diet in renal diseases; NET, neuroendocrine tumor; Neuroendocrine tumors; PPI, proton pump inhibitor; TRACE, time-resolved amplified cryptate emission; Temperature stability; Tumor markers.
Figures



Similar articles
-
Comparison of two chromogranin A assays and investigation of nonlinear specimens.Pract Lab Med. 2022 Aug 10;32:e00299. doi: 10.1016/j.plabm.2022.e00299. eCollection 2022 Nov. Pract Lab Med. 2022. PMID: 36035319 Free PMC article.
-
Automated two-site immunofluorescent assay for the measurement of serum chromogranin A.Clin Biochem. 2014 Jan;47(1-2):87-91. doi: 10.1016/j.clinbiochem.2013.10.029. Epub 2013 Nov 5. Clin Biochem. 2014. PMID: 24201067
-
Performance Evaluation of the KRYPTOR Compact PLUS Analyzer-Based B.R.A.H.M.S. CgA Ⅱ KRYPTOR Assay for Chromogranin A Measurement.Diagnostics (Basel). 2021 Dec 20;11(12):2400. doi: 10.3390/diagnostics11122400. Diagnostics (Basel). 2021. PMID: 34943638 Free PMC article.
-
Chromogranin A: any relevance in neuroendocrine tumors?Curr Opin Endocrinol Diabetes Obes. 2016 Feb;23(1):28-37. doi: 10.1097/MED.0000000000000215. Curr Opin Endocrinol Diabetes Obes. 2016. PMID: 26627724 Review.
-
[Chromogranin A (CgA) - the influence of various factors in vivo and in vitro, and existing disorders on it's concentration in blood].Endokrynol Pol. 2011;62 Suppl 1:25-8. Endokrynol Pol. 2011. PMID: 22125107 Review. Polish.
Cited by
-
Comparison of two chromogranin A assays and investigation of nonlinear specimens.Pract Lab Med. 2022 Aug 10;32:e00299. doi: 10.1016/j.plabm.2022.e00299. eCollection 2022 Nov. Pract Lab Med. 2022. PMID: 36035319 Free PMC article.
-
Prognostic value of chromogranin A in patients with GET/NEN in the pancreas and the small intestine.Endocr Connect. 2018 Jun;7(6):803-810. doi: 10.1530/EC-18-0059. Epub 2018 May 3. Endocr Connect. 2018. PMID: 29724794 Free PMC article.
-
Pitfalls in the Diagnosis and Management of Hypercortisolism (Cushing Syndrome) in Humans; A Review of the Laboratory Medicine Perspective.Diagnostics (Basel). 2023 Apr 14;13(8):1415. doi: 10.3390/diagnostics13081415. Diagnostics (Basel). 2023. PMID: 37189516 Free PMC article. Review.
-
Evaluation of CisBio ELISA for Chromogranin A Measurement.J Appl Lab Med. 2019 Jul;4(1):11-18. doi: 10.1373/jalm.2018.028027. Epub 2019 Apr 17. J Appl Lab Med. 2019. PMID: 31639703 Free PMC article.
-
Adrenal gland-released vasostatin-I is a myocardial depressant factor.Br J Clin Pharmacol. 2020 Apr;86(4):825-828. doi: 10.1111/bcp.14173. Epub 2020 Feb 3. Br J Clin Pharmacol. 2020. PMID: 31726481 Free PMC article.
References
-
- Modlin I.M., Gustafsson B.I., Moss S.F., Pavel M., Tsolakis A.V., Kidd M. Chromogranin A – biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol. 2010;17:2427–2443. - PubMed
-
- Vinik A.I., Silva M.P., Woltering E.A., Go V.L., Warner R., Caplin M. Biochemical testing for neuroendocrine tumors. Pancreas. 2009;38:876–889. - PubMed
-
- Nobels F.R., Kwekkeboom D.J., Coopmans W., Schoenmakers C.H., Lindemans J., De Herder W.W. Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron-specific enolase and the alpha-subunit of glycoprotein hormones. J Clin Endocrinol Metab. 1997;82:2622–2628. - PubMed
-
- de Herder W.W. Biochemistry of neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007;21:33–41. - PubMed
-
- Ramachandran R., Bech P., Murphy K.G., Dhillo W.S., Meeran K.M., Chapman R.S. Improved diagnostic accuracy for neuroendocrine neoplasms using two chromogranin A assays. Clin Endocrinol (Oxf) 2012;76:831–836. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

