A practical approach for the validation and clinical implementation of a high-sensitivity cardiac troponin I assay across a North American city
- PMID: 28932796
- PMCID: PMC5597710
- DOI: 10.1016/j.plabm.2015.02.001
A practical approach for the validation and clinical implementation of a high-sensitivity cardiac troponin I assay across a North American city
Abstract
Objectives: Despite several publications on the analytical performance of high-sensitivity cardiac troponin (hs-cTn) assays, there has been little information on how laboratories should validate and implement these assays into clinical service. Our study provides a practical approach for the validation and implementation of a hs-cTn assay across a large North American City.
Design and methods: Validation for the Abbott ARCHITECT hs-cTnI assay (across 5 analyzers) consisted of verification of limit of blank (LoB), precision (i.e., coefficient of variation; CV) testing at the reported limit of detection (LoD) and within and outside the 99th percentile, linearity testing, cTnI versus hs-cTnI patient comparison within and between analyzers (Passing and Bablok and non-parametric analyses). Education, clinical communications, and memorandums were issued in advance to inform all staff across the city as well as a selected reminder the day before live-date to important users. All hospitals switched to the hs-cTnI assay concurrently (the contemporary cTnI assay removed) with laboratory staff instructed to repeat samples previously measured with the contemporary cTnI assay with the hs-cTnI assay only by physician request.
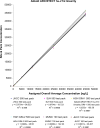
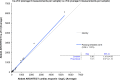
Results: Across the 5 analyzers and 6 reagent packs the overall LoB was 0.6 ng/L (n=60) with a CV of 33% at an overall mean of 1.2 ng/L (n=60; reported LoD=1.0 ng/L), with linearity demonstrated from 45,005 ng/L to 1.1 ng/L. Precision testing with a normal patient-pool QC material (mean range across 5 analyzers was 3.9-4.4 ng/L) yielded a range of CVs from 7% to 10% (within-run) and CVs from 7% to 18% (between-run) with the high patient-pool QC material (mean range across 5 analyzers was 29.6-36.3 ng/L) yielding a range of CVs from 2% to 5% (within-run) and CVs from 4% to 8% (between-run). There was agreement between hs-cTnI versus cTnI with the patient samples (slope ranges: 0.89-1.03; intercept ranges: 1.9-3.8 ng/L), however, the median CV on patient samples <100 ng/L across the analyzers was 5.6% for hs-cTnI versus 18.7% for the contemporary assay (p<0.001). Following the switch to hs-cTnI testing, no requests for repeat measurements were received.
Conclusions: Validation and implementation of hs-cTnI testing across multiple sites requires collaboration within the laboratories and between hospital laboratories and clinical staff.
Keywords: High-sensitivity cardiac troponin; Implementation; Multicenter; Quality; Validation.
Figures

Similar articles
-
Point-of-Care: Roadmap for Analytical Characterization and Validation of a High-Sensitivity Cardiac Troponin I Assay in Plasma and Whole Blood Matrices.J Appl Lab Med. 2022 Jun 30;7(4):971-988. doi: 10.1093/jalm/jfac028. J Appl Lab Med. 2022. PMID: 35660917
-
Analytical and clinical evaluation of an automated high-sensitivity cardiac troponin I assay for whole blood.Clin Chem Lab Med. 2025 Aug 15. doi: 10.1515/cclm-2025-0143. Online ahead of print. Clin Chem Lab Med. 2025. PMID: 40811682
-
Analytical characterization of the Siemens Dimension EXL high-sensitivity cardiac troponin I assay.Clin Biochem. 2019 Jul;69:52-56. doi: 10.1016/j.clinbiochem.2019.05.001. Epub 2019 May 4. Clin Biochem. 2019. PMID: 31063741
-
Transitioning high sensitivity cardiac troponin I (hs-cTnI) into routine diagnostic use: More than just a sensitivity issue.Pract Lab Med. 2016 Jan 13;4:62-75. doi: 10.1016/j.plabm.2016.01.001. eCollection 2016 Apr 1. Pract Lab Med. 2016. PMID: 28856194 Free PMC article.
-
High-sensitivity cardiac troponin assays: From improved analytical performance to enhanced risk stratification.Crit Rev Clin Lab Sci. 2017 May;54(3):143-172. doi: 10.1080/10408363.2017.1285268. Epub 2017 May 1. Crit Rev Clin Lab Sci. 2017. PMID: 28457177 Review.
Cited by
-
Acute Phase Response and Non-Reproducible Elevated Concentrations with a High-Sensitivity Cardiac Troponin I Assay.J Clin Med. 2021 Mar 2;10(5):1014. doi: 10.3390/jcm10051014. J Clin Med. 2021. PMID: 33801415 Free PMC article.
-
Analytical validation of cardiac troponin I assays in horses.J Vet Diagn Invest. 2018 Mar;30(2):226-232. doi: 10.1177/1040638717747070. Epub 2017 Dec 10. J Vet Diagn Invest. 2018. PMID: 29224512 Free PMC article.
-
Undetectable Concentrations of a Food and Drug Administration-approved High-sensitivity Cardiac Troponin T Assay to Rule Out Acute Myocardial Infarction at Emergency Department Arrival.Acad Emerg Med. 2017 Oct;24(10):1267-1277. doi: 10.1111/acem.13229. Epub 2017 Aug 11. Acad Emerg Med. 2017. PMID: 28544100 Free PMC article.
-
Prevalence of Positive Troponin and Echocardiogram Findings and Association With Mortality in Acute Ischemic Stroke.Stroke. 2017 May;48(5):1226-1232. doi: 10.1161/STROKEAHA.116.014561. Epub 2017 Apr 5. Stroke. 2017. PMID: 28381647 Free PMC article.
-
Clinical chemistry score versus high-sensitivity cardiac troponin I and T tests alone to identify patients at low or high risk for myocardial infarction or death at presentation to the emergency department.CMAJ. 2018 Aug 20;190(33):E974-E984. doi: 10.1503/cmaj.180144. CMAJ. 2018. PMID: 30127037 Free PMC article.
References
-
- Kavsak P.A. Early standardization of high sensitivity troponin T reporting – a lost opportunity. Clin Biochem. 2011;44:758–759. - PubMed
-
- High-sensitivity cardiac troponin for the rapid diagnosis of acute coronary syndrome in the emergency department: a clinical and cost-effectiveness evaluation [Internet]. Source: Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; March 2013. 〈http://www.cadth.ca/en/products/optimal-use/ high-sensitivity-troponin〉. - PubMed
-
- Kavsak P.A., Jaffe A.S., Hickman P.E., Mills N.L., Humphries K.H., McRae A. Canadian Institutes of Health Research dissemination grant on high-sensitivity cardiac troponin. Clin Biochem. 2014;47:155–157. - PubMed
-
- Kavsak P.A., Allen L.C., Apple F.S., Booth R.A., Chan P.C., Delvin E. Cardiac troponin testing in the acute care setting: ordering, reporting, and high sensitivity assays—an update from the Canadian society of clinical chemists (CSCC) Clin Biochem. 2011;44:1273–1277. - PubMed
-
- Apple F.S., Collinson P.O., IFCC Task Force on Clinical Applications of Cardiac Biomarkers Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem. 2012;58:54–61. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

