Multicenter comparison of automated procalcitonin immunoassays
- PMID: 28932801
- PMCID: PMC5597721
- DOI: 10.1016/j.plabm.2015.07.001
Multicenter comparison of automated procalcitonin immunoassays
Abstract
Objectives: A multicenter study to compare results of BRAHMS Kryptor PCT with those obtained using four BRAHMS-partnered procalcitonin (PCT) automated immunoassays (DiaSorin Liaison, BioMérieux Vidas, Roche Cobas E601 and Siemens Advia Centaur) and the Diazyme immunotubidimetric assay implemented on four clinical chemistry platforms (Abbott Architect c16000, Siemens Advia 2400, Roche Cobas C501 and Beckman Coulter AU5800).
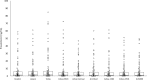
Design and methods: One hundred serum samples from in-patients with PCT values between 0.10 and 58.7 ng/mL were divided into aliquots and tested with the nine different reagents and analyzers. BRAHMS PCT Kryptor results were used as reference.
Results: Compared to BRAHMS PCT Kryptor, significant differences in results were observed on Vidas, Advia Centaur, Architect, Cobas C501 and AU5800. However, the correlation coeffiecients (r) with BRAHMS PCT Kryptor were between 0.899 and 0.988. The mean bias was less than ±1.02 ng/mL, except for Vidas (2.70 ng/mL). The agreement at three clinically relevant cut-offs was optimal: between 83-98% at 0.50 ng/mL, 90-97% at 2.0 ng/mL, and 98% at 10 ng/mL. The comparison of Diazyme PCT across the four clinical chemistry analyzers yielded high correlation coefficients (r between 0.952 and 0.976), a mean bias less than ±0.9 ng/mL, acceptable agreement at 0.5 ng/mL (>82%), and high concordance at the 2.0 ng/mL (>97%) and 10 ng/mL (>98%) cut-offs.
Conclusions: The methods and applications evaluated in this multicenter study are aligned with BRAHMS PCT Kryptor and can be used for predicting the risk of progression to systemic inflammation in patients with bacterial infections using the conventional PCT diagnostic thresholds.
Keywords: Bacterial infections; Immunoassay; Multicenter study; Procalcitonin; Sepsis.
Figures

References
-
- Meisner M. 1st ed. UNI-MED; Bremen: 2010. Procalcitonin-Biochemistry and Clinical Diagnosis. ISBN 978-3-8374-1241-3.
-
- Lippi G., Danese E., Cervellin G., Montagnana M. Laboratory diagnostics of spontaneous bacterial peritonitis. Clin. Chim. Acta. 2014;430:164–170. - PubMed
-
- Lippi G., Meschi T., Cervellin G. Inflammatory biomarkers for the diagnosis, monitoring and follow-up of community-acquired pneumonia: clinical evidence and perspectives. Eur. J. Intern. Med. 2011;22:460–465. - PubMed
-
- Soni N.J., Samson D.J., Galaydick J.L., Vats V., Pitrak D.L., Aronson N. Agency for Healthcare Research and Quality (US); Rockville (MD): 2012. Procalcitonin-Guided Antibiotic Therapy. October. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

