14-3-3 Regulates Actin Filament Formation in the Deep-Branching Eukaryote Giardia lamblia
- PMID: 28932813
- PMCID: PMC5597967
- DOI: 10.1128/mSphere.00248-17
14-3-3 Regulates Actin Filament Formation in the Deep-Branching Eukaryote Giardia lamblia
Abstract
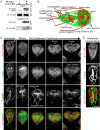
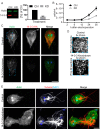
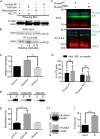
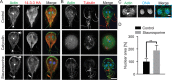
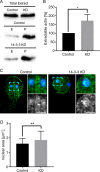
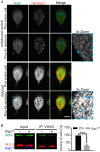
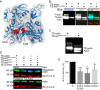
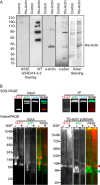
The phosphoserine/phosphothreonine-binding protein 14-3-3 is known to regulate actin; this function has been previously attributed to sequestration of phosphorylated cofilin. 14-3-3 was identified as an actin-associated protein in the deep-branching eukaryote Giardia lamblia; however, Giardia lacks cofilin and all other canonical actin-binding proteins (ABPs). Thus, the role of G. lamblia 14-3-3 (Gl-14-3-3) in actin regulation was unknown. Gl-14-3-3 depletion resulted in an overall disruption of actin organization characterized by ectopically distributed short actin filaments. Using phosphatase and kinase inhibitors, we demonstrated that actin phosphorylation correlated with destabilization of the actin network and increased complex formation with 14-3-3, while blocking actin phosphorylation stabilized actin filaments and attenuated complex formation. Giardia's sole Rho family GTPase, Gl-Rac, modulates Gl-14-3-3's association with actin, providing the first connection between Gl-Rac and the actin cytoskeleton in Giardia. Giardia actin (Gl-actin) contains two putative 14-3-3 binding motifs, one of which (S330) is conserved in mammalian actin. Mutation of these sites reduced, but did not completely disrupt, the association with 14-3-3. Native gels and overlay assays indicate that intermediate proteins are required to support complex formation between 14-3-3 and actin. Overall, our results support a role for 14-3-3 as a regulator of actin; however, the presence of multiple 14-3-3-actin complexes suggests a more complex regulatory relationship than might be expected for a minimalistic parasite. IMPORTANCEGiardia lacks canonical actin-binding proteins. Gl-14-3-3 was identified as an actin interactor, but the significance of this interaction was unknown. Loss of Gl-14-3-3 results in ectopic short actin filaments, indicating that Gl-14-3-3 is an important regulator of the actin cytoskeleton in Giardia. Drug studies indicate that Gl-14-3-3 complex formation is in part phospho-regulated. We demonstrate that complex formation is downstream of Giardia's sole Rho family GTPase, Gl-Rac. This result provides the first mechanistic connection between Gl-Rac and Gl-actin in Giardia. Native gels and overlay assays indicate intermediate proteins are required to support the interaction between Gl-14-3-3 and Gl-actin, suggesting that Gl-14-3-3 is regulating multiple Gl-actin complexes.
Keywords: 14-3-3; Rho GTPase; actin; evolutionary cell biology.
Figures
Similar articles
-
Identification of Actin Filament-Associated Proteins in Giardia lamblia.Microbiol Spectr. 2021 Sep 3;9(1):e0055821. doi: 10.1128/Spectrum.00558-21. Epub 2021 Jul 21. Microbiol Spectr. 2021. PMID: 34287056 Free PMC article.
-
Rac Regulates Giardia lamblia Encystation by Coordinating Cyst Wall Protein Trafficking and Secretion.mBio. 2016 Aug 23;7(4):e01003-16. doi: 10.1128/mBio.01003-16. mBio. 2016. PMID: 27555307 Free PMC article.
-
An actin cytoskeleton with evolutionarily conserved functions in the absence of canonical actin-binding proteins.Proc Natl Acad Sci U S A. 2011 Apr 12;108(15):6151-6. doi: 10.1073/pnas.1018593108. Epub 2011 Mar 28. Proc Natl Acad Sci U S A. 2011. PMID: 21444821 Free PMC article.
-
Microtubule organelles in Giardia.Adv Parasitol. 2020;107:25-96. doi: 10.1016/bs.apar.2019.11.001. Epub 2020 Feb 5. Adv Parasitol. 2020. PMID: 32122531 Free PMC article. Review.
-
Rho-associated coiled-coil kinase (ROCK) signaling and disease.Crit Rev Biochem Mol Biol. 2013 Jul-Aug;48(4):301-16. doi: 10.3109/10409238.2013.786671. Epub 2013 Apr 19. Crit Rev Biochem Mol Biol. 2013. PMID: 23601011 Review.
Cited by
-
The Giardia ventrolateral flange is a lamellar membrane protrusion that supports attachment.PLoS Pathog. 2022 Apr 28;18(4):e1010496. doi: 10.1371/journal.ppat.1010496. eCollection 2022 Apr. PLoS Pathog. 2022. PMID: 35482847 Free PMC article.
-
EhP3, a homolog of 14-3-3 family of protein participates in actin reorganization and phagocytosis in Entamoeba histolytica.PLoS Pathog. 2019 May 16;15(5):e1007789. doi: 10.1371/journal.ppat.1007789. eCollection 2019 May. PLoS Pathog. 2019. PMID: 31095644 Free PMC article.
-
Regulation of the Actin Cytoskeleton via Rho GTPase Signalling in Dictyostelium and Mammalian Cells: A Parallel Slalom.Cells. 2021 Jun 24;10(7):1592. doi: 10.3390/cells10071592. Cells. 2021. PMID: 34202767 Free PMC article. Review.
-
Omics Analyses of Trichomonas vaginalis Actin and Tubulin and Their Participation in Intercellular Interactions and Cytokinesis.Genes (Basel). 2022 Jun 15;13(6):1067. doi: 10.3390/genes13061067. Genes (Basel). 2022. PMID: 35741829 Free PMC article.
-
Differential Subcellular Distribution and Translocation of Seven 14-3-3 Isoforms in Response to EGF and During the Cell Cycle.Int J Mol Sci. 2020 Jan 2;21(1):318. doi: 10.3390/ijms21010318. Int J Mol Sci. 2020. PMID: 31906564 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

