Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles
- PMID: 28932818
- PMCID: PMC5579732
- DOI: 10.1002/btm2.10065
Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles
Abstract
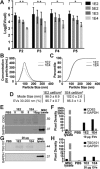
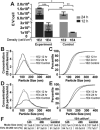
Mesenchymal stem cell (MSC)-derived extracellular vesicles (EVs) have emerged as potential therapeutic agents for numerous applications. EVs offer potential advantages over cell-based therapies with regard to safety, stability and clearance profiles, however production and potency limitations must be addressed to enable eventual translation of EV-based approaches. Thus, we sought to examine the role of specific cell culture parameters on MSC EV production and bioactivity toward informing rational design parameters for scalable EV biomanufacturing. We report significantly reduced MSC EV vascularization bioactivity, as measured by an endothelial cell gap closure assay, with increasing passage in culture by trypsinization, especially beyond passage 4. We further show that increased frequency of EV collection yielded higher numbers of EVs from the same initial number of MSCs over a 24 hr period. Finally, we demonstrate that decreased cell seeding density in culture flasks resulted in increased production of EVs per cell in MSCs and other cell types. Overall, these studies highlight the need for careful consideration of the parameters of cell passage number and cell seeding density in the production of therapeutic EVs at laboratory scale and for rational design of large-scale EV biomanufacturing schemes.
Keywords: EVs; biomanufacturing; exosomes; mesenchymal stem cells; therapeutic angiogenesis.
Figures





References
-
- Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–1219. doi:10.1161/CIRCRESAHA.108.176826. - DOI - PMC - PubMed
-
- Gnecchi M, Danieli P, Malpasso G, Ciuffreda MC. Paracrine mechanisms of mesenchymal stem cells in tissue repair In: Gnecchi M, ed. Mesenchymal Stem Cells: Methods and Protocols. New York, NY: Springer New York; 2016:123–146. doi:10.1007/978-1-4939-3584-0_7. - DOI - PubMed
-
- Huang S, Wu Y, Gao D, Fu X. Paracrine action of mesenchymal stromal cells delivered by microspheres contributes to cutaneous wound healing and prevents scar formation in mice. Cytotherapy. 2017;17(7):922–931. doi:10.1016/j.jcyt.2015.03.690. - DOI - PubMed
-
- Hwang H, Kloner RA. Improving regenerating potential of the heart after myocardial infarction: factor‐based approach. Life Sci. 2010;86(13–14):461–472. doi:10.1016/j.lfs.2010.01.004. - DOI - PubMed
-
- Simons M, Annex BH, Laham RJ, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor‐2. Circulation. 2002;105(7):788–793. doi:10.1161/hc0802.104407. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

