Factor VIIa interaction with EPCR modulates the hemostatic effect of rFVIIa in hemophilia therapy: Mode of its action
- PMID: 28932824
- PMCID: PMC5602564
- DOI: 10.1182/bloodadvances.2016004143
Factor VIIa interaction with EPCR modulates the hemostatic effect of rFVIIa in hemophilia therapy: Mode of its action
Abstract
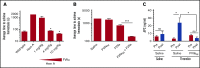
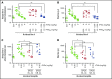
Recent studies established that clotting factor VIIa (FVIIa) binds endothelial cell protein C receptor (EPCR). It has been speculated that FVIIa interaction with EPCR might augment the hemostatic effect of rFVIIa in therapeutic conditions. The present study is carried out to investigate the mechanism by which FVIIa interaction with EPCR contributes to the hemostatic effect of rFVIIa in hemophilia therapy. Active-site inhibited FVIIa, which is capable of binding to EPCR but has no ability to activate factor X, reduced the concentration of rFVIIa required to correct the bleeding following the saphenous vein injury in mouse hemophilia model systems. Higher doses of rFVIIa were required to restore hemostasis in EPCR overexpressing hemophilia mice compared to hemophilia mice expressing normal levels of EPCR. Administration of FVIII antibody induced only mild hemophilic bleeding in EPCR-deficient mice, which was corrected completely with a low dose of rFVIIa. Administration of therapeutic concentrations of rFVIIa increased plasma protein C levels in EPCR overexpressing mice, indicating the displacement of protein C from EPCR by rFVIIa. EPCR levels did not significantly alter the bioavailability of rFVIIa in plasma. Overall, our data indicate that EPCR levels influence the hemostatic effect of rFVIIa in treating hemophilia. Our present findings suggest that FVIIa displacement of anticoagulant protein C from EPCR that results in down-regulation of activated protein C generation and not the direct effect of EPCR-FVIIa on FX activation is the mechanism by which FVIIa interaction with EPCR contributes to the hemostatic effect of rFVIIa in hemophilia therapy.
Conflict of interest statement
Conflict-of-interest The authors declare no competing financial interests.
Figures




Similar articles
-
Factor VIIa induces extracellular vesicles from the endothelium: a potential mechanism for its hemostatic effect.Blood. 2021 Jun 17;137(24):3428-3442. doi: 10.1182/blood.2020008417. Blood. 2021. PMID: 33534910 Free PMC article.
-
EPCR deficiency or function-blocking antibody protects against joint bleeding-induced pathology in hemophilia mice.Blood. 2020 Jun 18;135(25):2211-2223. doi: 10.1182/blood.2019003824. Blood. 2020. PMID: 32294155 Free PMC article.
-
One amino acid in mouse activated factor VII defines its endothelial protein C receptor (EPCR) binding and modulates its EPCR-dependent hemostatic activity in vivo.J Thromb Haemost. 2017 Mar;15(3):507-512. doi: 10.1111/jth.13607. Epub 2017 Feb 14. J Thromb Haemost. 2017. PMID: 28035745
-
Potential role of recombinant factor VIIa as a hemostatic agent.Clin Adv Hematol Oncol. 2003 Feb;1(2):112-9. Clin Adv Hematol Oncol. 2003. PMID: 16224390 Review.
-
Recombinant factor VIIa: new insights into the mechanism of action through product innovation.Res Pract Thromb Haemost. 2024 Dec 31;9(1):102670. doi: 10.1016/j.rpth.2024.102670. eCollection 2025 Jan. Res Pract Thromb Haemost. 2024. PMID: 39990097 Free PMC article. Review.
Cited by
-
Selective inhibition of activated protein C anticoagulant activity protects against hemophilic arthropathy in mice.Blood. 2022 May 5;139(18):2830-2841. doi: 10.1182/blood.2021013119. Blood. 2022. PMID: 35143636 Free PMC article.
-
The safety of activated eptacog beta in the management of bleeding episodes and perioperative haemostasis in adult and paediatric haemophilia patients with inhibitors.Haemophilia. 2021 Nov;27(6):921-931. doi: 10.1111/hae.14419. Epub 2021 Oct 11. Haemophilia. 2021. PMID: 34636112 Free PMC article. Clinical Trial.
-
Factor VIIa induces anti-inflammatory signaling via EPCR and PAR1.Blood. 2018 May 24;131(21):2379-2392. doi: 10.1182/blood-2017-10-813527. Epub 2018 Apr 18. Blood. 2018. PMID: 29669778 Free PMC article.
-
Endothelial Protein C Receptor and Its Impact on Rheumatic Disease.J Clin Med. 2024 Mar 31;13(7):2030. doi: 10.3390/jcm13072030. J Clin Med. 2024. PMID: 38610795 Free PMC article. Review.
-
Factor VIIa induces extracellular vesicles from the endothelium: a potential mechanism for its hemostatic effect.Blood. 2021 Jun 17;137(24):3428-3442. doi: 10.1182/blood.2020008417. Blood. 2021. PMID: 33534910 Free PMC article.
References
-
- Preston RJ, Ajzner E, Razzari C, et al. . Multifunctional specificity of the protein C/activated protein C Gla domain. J Biol Chem. 2006;281(39):28850-28857. - PubMed
-
- López-Sagaseta J, Montes R, Puy C, Díez N, Fukudome K, Hermida J. Binding of factor VIIa to the endothelial cell protein C receptor reduces its coagulant activity. J Thromb Haemost. 2007;5(9):1817-1824. - PubMed
-
- Rapaport SI, Rao LVM. The tissue factor pathway: how it has become a “prima ballerina.” Thromb Haemost. 1995;74(1):7-17. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

