Helicase-dependent isothermal amplification: a novel tool in the development of molecular-based analytical systems for rapid pathogen detection
- PMID: 28932883
- PMCID: PMC7079856
- DOI: 10.1007/s00216-017-0620-3
Helicase-dependent isothermal amplification: a novel tool in the development of molecular-based analytical systems for rapid pathogen detection
Abstract
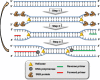
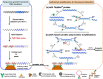
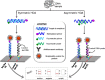
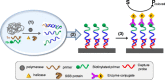
Highly sensitive testing of nucleic acids is essential to improve the detection of pathogens, which pose a major threat for public health worldwide. Currently available molecular assays, mainly based on PCR, have a limited utility in point-of-need control or resource-limited settings. Consequently, there is a strong interest in developing cost-effective, robust, and portable platforms for early detection of these harmful microorganisms. Since its description in 2004, isothermal helicase-dependent amplification (HDA) has been successfully applied in the development of novel molecular-based technologies for rapid, sensitive, and selective detection of viruses and bacteria. In this review, we highlight relevant analytical systems using this simple nucleic acid amplification methodology that takes place at a constant temperature and that is readily compatible with microfluidic technologies. Different strategies for monitoring HDA amplification products are described. In addition, we present technological advances for integrating sample preparation, HDA amplification, and detection. Future perspectives and challenges toward point-of-need use not only for clinical diagnosis but also in food safety testing and environmental monitoring are also discussed. Graphical Abstract Expanding the analytical toolbox for the detection of DNA sequences specific of pathogens with isothermal helicase dependent amplification (HDA).
Keywords: Helicase; Isothermal; Molecular test; Nucleic acid amplification; Pathogen detection.
Conflict of interest statement
The authors declare that they have no conflict of interest
Figures

References
-
- Disease outbreaks news. www.who.int/csr/don/en. Accessed 1 June 2017.
-
- Saker L, Lee K, Cannito B, Gilmore A, Campbell-Lendrum D. Globalization and infectious diseases: a review of the linkages. Special Topics No. 3. UNICEF/UNDP/World Bank/WHO 2004; 1–67
-
- Accessible quality-assured diagnostics. 2009 annual report. Available from: www.who.int/tdr/publications/about-tdr/annual-reports/bl7-annual-report/en/. Accessed 1 June 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

